Safique Alam
Junior Research Fellow
Email:- safique.sa@gmail.com
Biotech Hub, D.R. College, Golaghat, Assam
DNA is the essential carrier of genetic information in the living organisms. Though sturdy, DNA undergoes spontaneous modifications naturally as a result of metabolic or hydrolytic processes. Any damage to DNA structure can lead to mutations and genetic instability. Paraphrased, DNA damage is the alteration in the chemical structure of DNA such as a base missing from the backbone of DNA, break in the strand, or a chemically changed base. DNA damage is distinctly different from mutation although both are types of error in DNA. DNA damage is an abnormal chemical structure in DNA, while a mutation is a change in the sequence of standard base pairs. DNA damage may also be associated with Type 2 Diabetes Mellitus; its complications are mainly through oxidative stress. Oxidative stress is known to increase the conversion of deoxyguanosine to 8-oxo,2′-deoxyguanosine in DNA. To investigate the possible contribution of oxidative DNA damage to the pathogenesis of diabetic complications, measurement of the content of 8-oxo,2′-deoxyguanosine in the urine and the blood mononuclear cells of Type 2 (non-insulin-dependent) diabetic patients is done.
Diabetes Mellitus is a metabolic syndrome of disordered metabolism, usually due to a combination of hereditary and environmental causes, resulting in abnormally high blood sugar levels (hyperglycaemia). World Health Organisation (WHO) defined diabetes mellitus as a heterogeneous metabolic disorder characterised by common feature of chronic hyperglycaemia, which can lead to serious damage to the heart, blood vessels, nerves, kidneys and eyes. Diabetes is an important public health problem. Over the past three decades, diabetes has become a major cause for morbidity and mortality affecting the youth and mainly middle-aged. Although the prevalence of type 1 diabetes (juvenile diabetes or insulin-dependent diabetes) is also increasing, type 2 diabetes accounts for more than 90% of all the diabetes cases. In 2000, WHO reported that at least 171 million people worldwide suffer from diabetes 1, i.e. 2.8% of the population. According to 2014 data from WHO, diabetes has affected approximately 422 million people worldwide; 1.5 million deaths caused (2012 reports). The largest increase of diabetic population occurs in most economically productive age groups; it emerged as a very serious health problem for the citizens of developed countries. The incidence now risen dramatically also in the developing countries.
Diabetes is characterised by either Type 1 Diabetes Mellitus (T1DM), also called Insulin Dependent Diabetes Mellitus (IDDM) caused by lack of insulin secretion by β-cells of the pancreas and Type 2 Diabetes Mellitus (T2DM) also called Non-Insulin Dependent Diabetes Mellitus (NIDDM) caused by decreased sensitivity of target tissues to insulin. The classic signs and symptoms of diabetes are polyuria, polydipsia, fatigue, sweating, weight loss etc. Diabetes mellitus type 2 (T2DM) with its resulting complications is one of the biggest preventable health problems of the 21st century and has developed to a major challenge for the health system as one of the fastest increasing diseases worldwide. T2DM develops mostly undiagnosed in overweight individuals as a result of pancreatic ß-cell dysfunction and impaired glucose tolerance. If left untreated, the underlying hyperglycaemia leads to a ß-cell failure and an indispensable need for exogenous insulin supply. Hyperglycemia is usually measured by the percentage of glycated hemoglobin (HbA1c) from the total amount of blood hemoglobin, which evolves through a long-term exposure to elevated glucose in the blood stream. Chronic hyperglycemia promotes oxidative stress, which represents a major patho-physiological link between progression of T2DM and the onset of severe diabetic complications such as diabetic foot ulcers, myocardial infarction or cerebro-vascular accidents. Especially, cardiovascular events lead to premature mortality in diabetes patients. Oxidative stress can trigger damage to DNA which has been linked to enhanced cancer risk. Therefore, patients with diabetes mellitus show an increased cancer incidence, with a strong linear association between HbA1c levels and gastric, pancreatic, colorectal, breast and live cancer incidence. The major risk factors associated with the disease are: having a family history of diabetes, being over the age of 40, being overweight, having low HDL cholesterol, having a history if gestational diabetes, giving birth to a baby weighing 9 pounds or more, hypertension, smoking, dietary pattern and recently also specific genes are identified as one of the most documented risk factors for T2DM. The occurrence of free radical induced lipid peroxidation, owing to free radical activity, plays an important role in the development of complications of diabetes. The existence of hyperglycemia produces increased oxidative stress (OS) via non-enzymatic glycation, glucose autoxidation, and alterations in polyol pathway activity with subsequent influences on the whole organism. As oxidative stress impairs not only lipids and proteins, but also DNA; the level of DNA strand break can be studied using comet assay modifications.
Comet Assay
Single cell gel electrophoresis (SCGE) or the Comet Assay is a versatile, sensitive yet simple and economical technique used to measure DNA damage and repair in individual cells. The comet assay helps to measure the single/double-strand DNA breaks, alkali labile sites (apurinic/apyrimidinic sites), DNA cross-links, base/base-pair damages and apoptotic nuclei in the cells. It is a convenient tool for measuring universal DNA damage in individual cells. DNA damage, due to environmental factors and normal metabolic processes inside the cell, occurs at a rate of 1000 to 1,000,000 molecular lesions per day. While this counts for only a small part of the human genomes of approximately 6 billion bases, unrepaired lesions to critical genes can impede a cells ability to carry out its function and increase the likelihood of cancer.
To perform Comet assay, firstly the individual cells are mixed with molten agarose before application to the comet slide. These embedded cells are then treated with a lysis buffer and alkaline solution, which relaxes and denatures the DNA. Finally, the samples are electrophoresed in a horizontal chamber to separate intact DNA from damaged fragments. Following electrophoresis, the samples are dried, stained with a DNA dye and visualized by epifluorescence microscopy. Under these conditions, the damaged DNA migrates further than intact DNA and produce a “comet tail” shape.
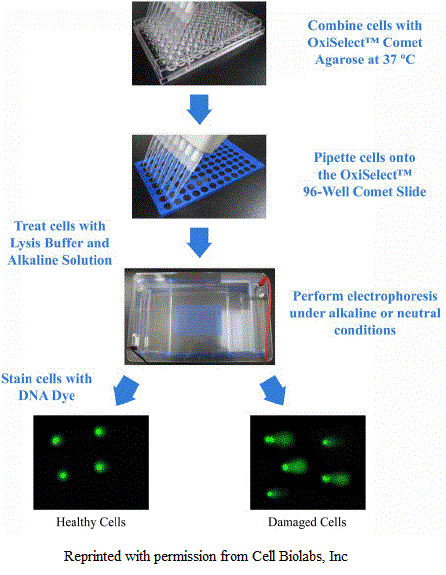
Figure: Representation of Comet Assay.
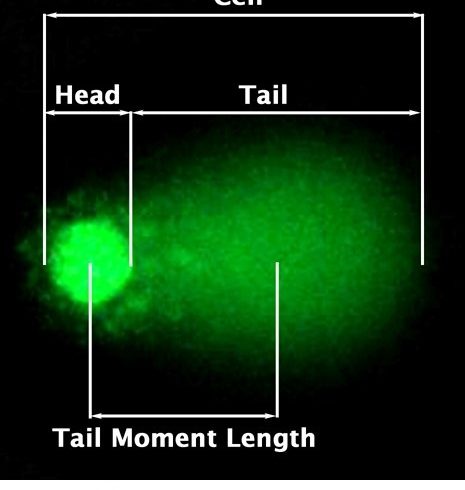
The DNA damage is quantified by measuring the displacement between the genetic material of the nucleus (comet head) and resulting ‘tail’. Tail Moment and Tail DNA% are two most common parameters to analyse Comet assay results. At least 50-100 cells should be analysed per sample.
Tail DNA% = 100 x Tail DNA Intensity/ Cell DNA Intensity
Tail Moment can be measured using one of the following methods:
- (a) Olive Tail Moment:- Tail DNA% x Tail Moment Length
- (b) Extent Tail Moment:- Tail DNA% x Length Of Tail
*Tail Moment Length is measured from the centre of the head to the centre of the tail.
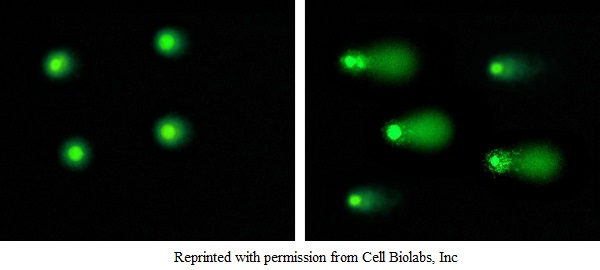
Healthy cells Damaged cells
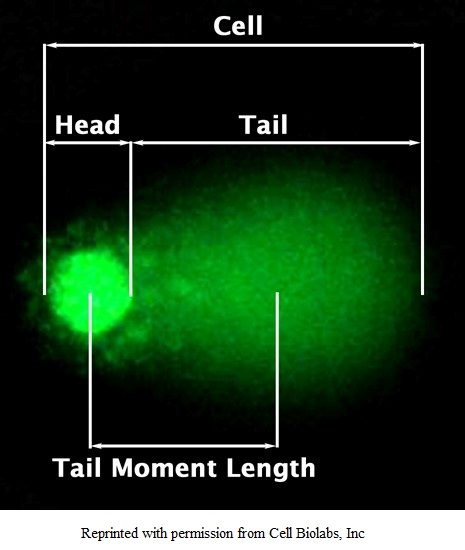
Figure: Typical Damaged DNA in Comet Assay.
Discussion:
Diabetes mellitus is a chronic disease, for which there is no known cure except in very specific situations. Its management concentrates on keeping blood sugar levels as close to normal as possible without causing hypoglycaemia. This can usually be accomplished with diet control, exercise and use of appropriate medications, and learning about the disease and actively participating in the treatment for people with diabetes. Good metabolic control of hyperglycaemia will prevent in alteration in peroxidation and the lipid metabolism, which may help in good prognosis and preventing manifestation of vascular and secondary complication in diabetes mellitus. Diabetes of all types can lead to complications in many parts of the body and can increase the overall risk of dying prematurely. Possible complications include heart attack, stroke, kidney failure, leg amputation, vision loss and nerve damage. In pregnancy, poorly controlled diabetes increases the risk of foetal death and other complications.
References:
Wild, S.; Roglic, G.; Green, A.; Sicree, R. and King, H. (2004), “Global Prevalence of diabetic: estimate for the year 2000 and projection for 2030”. Diabetes Care 27(5): 1045-53.
Buse, J.B.; Polonsky, K.S. and Burant, C.F. (2011). Type 2 Diabetes Mellitus. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier-Saunders. pp. 1371 – 435.
Leinonen, J.; Lehtimaki, T.; Toyokuni, S. et al. (1997) New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent diabetes mellitus. FEBS Letters 417: 150 – 152.
Hinokio, Y.; Suzuki, S.; Hirai, M.; Chiba, M.; Hirai, A. and Toyota, T. (1999). Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia, 42:995-998.
De Boeck, M.; Touil, N.; De Visscher, G.; Vande, P.A. and Kirsch-Volders, M. (2000). Validation and implementation of an internal standard in Comet assay. Mutat. Res. 469: 181-197.