Madhusmita Dehingia1 and Mojibur R. Khan2
1DBT-RA, Dept. of Animal Biotechnology, College of Veterinary Science, AAU, Khanapara
2Assistant Professor, Division of Life Sciences, IASST, Guwahati
Trillions of microbes, called ‘microbiota’ colonize the human body and have co-evolved with the host over millions of years. It has been estimated that the human microbiota is composed of over 35,000 bacterial species and the human microbiome (collective genomes of microbiota) contains ~150 times more genes than the human genome. Variation in microbiota composition is observed across body sites and across individuals. The gastrointestinal tract, oral cavity, skin, airways and vagina are the well characterized sites of microbial colonization. Each body site is colonized by a characteristic group of microbes with variable abundance which is relatively stable across individuals and over time. The GI tract is the most heavily colonized organ, while colon alone contains 70% of the microbes present in the human body. Gut bacteria play an important role in host nutrient metabolism by metabolizing the undigested cellulose and the resistant starch which are otherwise inaccessible to the host. By this process, gut bacteria contribute upto 10% of the daily energy uptake by synthesizing products such as short chain fatty acids (SCFAs). Gut bacteria produce essential amino acids, vitamins and are involved in metabolism of drugs and detoxification of potentially harmful xenobiotics. Other than the role in nutrient metabolism and cellular uptakes, the gut bacteria also play an important role in shaping the immune system. Germ free mice (GF) (that are devoid of any microbes) were reported to have defective lymphoid structures, spleen and lymph nodes. Reduced levels of helper T cells and cytotoxic T cells as well as reduced levels of immunoglobulins (both IgA and IgG) were reported in GF mice. Furthermore, gut microbiota also has a barrier effect that protects the host gut from pathogen invasion by colonizing the mucosal surfaces. Majority (99.9%) of the human gut microbiota are strict anaerobes and are thousand times more dominant than the aerobes. Bacteroidetes and Firmicutes are the two major bacterial phyla of the human digestive tract while Proteobacteria, Actinobacteria, Verrucomicrobia, Fusobacteria and Cyanobacteria are present in minor proportions.
Among the many microbial communities of the human body, gut microbes play a crucial role in influencing health and diseases. The number of diseases claimed to be driven by gut microbial dysbiosis has grown exponentially which includes allergy, asthma, inflammatory bowel diseases, diabetes, obesity etc. Growing evidences indicate the role of gut microbiota in regulating brain development, function and behaviour. Reports have shown the role of gut microbiota in the development of psychiatric diseases such as Parkinson’s disease, autism spectrum disorder (ASD’s), disorder of moods etc. Preliminary studies have shown the differences in the composition of gut microbiota in patients with depression compared to healthy individuals. Transplantation of faecal samples pooled from five patients with depression into germ-free mice resulted in depressive behavioural patterns, while faecal transplant from healthy individuals had no behavioural effect. Though the study on germ-free mice provided the evidence of transfer of depressive phenotype by microbiota transplantation, germ-free mice are known to be immune deficient. Therefore, a similar study was repeated in microbiota deficient mice model with well-developed immune system and normal brain function. Faecal transplantation from the patients with depression into this model induced depression which includes anhedonia (inability to feel pleasure in normally pleasurable activities), anxiety like behaviour and alteration in tryptophan metabolism. This study provided convincing evidence that the gut microbiota have a causal role in development of depressive behaviour and could serve as a target for treatment and prevention of the disorder. Maternal high fat diet (MHFD) induce obesity in the offspring which can also lead to the development of social behavioural deficit and neuro-developmental disorders such as autism spectrum disorder which are reported to be mediated by alteration in the offspring’s gut microbiota. Metagenomic study revealed the decreased abundance of several bacterial species with a notable reduction in Lactobacillus reuteri in the gut of MHFD offspring compared to animals with normal diet. MHFD induced changes in the gut microbiota of the offspring were reported to be associated with the major alteration in the mesolimbic dopamine reward system within the ventral tegmental area. When the offsprings were treated with a commensal Lactobacillus reuteri strain 6475, it increased the oxytocin level (known to influence social behaviour) which ameliorated the synaptic dysfunction in the ventral tegmental area and reversed the social behaviour in the MHFD. Commensal Lactobacillus species (L. rhamnosus) can influence the vagus nerve and can bring behavioural changes and also changes in the central γ-aminobutyric acid (GABA) receptor expression. The L. reuteri 6475 strain also has anti-inflammatory activity in vivo as well as in vitro that converts L-histidine to immunoregulatory mediator histamine. Obesity is also reported to be associated with systematic inflammation which can affect the development of foetal brain. Brain behaviour and function are also influenced by microbial metabolites such as acetate, propionate and butyrate which are key products of gut microbiota. Increased production of acetate by gut microbiota in rodents can activate the parasympathetic nervous system, which in turn promotes increased glucose stimulated insulin secretion, hyperphagia and obesity. This evidence supports the previous report suggesting the role of gut microbiota in social behaviour.
There exists a bidirectional signalling between the gastrointestinal tract and the brain which involves gut microbiota. This relationship is called as the gut-brain axis or microbiota-gut-brain axis which involves different pathways such as the vagus nerve and the hypothalamic- pituitary- adrenal pathway that maintains homeostasis in satiety and hunger (Figure 1). Now, one may think, whether we can control our hunger and satiety! Scientists had made this possible in genetically engineered mice in which a magnetic stimulation was used to switch on neurons in the ventro-medial hypothalamus region of the brain which increased the blood sugar levels in the rodents and decreased the level of insulin. As a result, the mice ate more than their control counterparts, while inhibition of the neurons had the opposite effects, such as drop in blood sugar level, elevated insulin level and suppressed hunger. Thus, the communication between the gut and brain happens not only through nerve connections between the organs but also through biochemical signals, such as hormones. The gut-brain axis seems to influence a range of diseases. Therefore, scientists have begun to concentrate on the central nervous system (CNS) and the enteric nervous system (ENS) in order to treat certain metabolic disorders like obesity and diabetes. Obesity and diabetes are two major health problems for the people worldwide. Gastric bypass and bariatric surgery are two well accepted treatments for people with obesity and diabetes as these procedures elevate the level of peptide YY (PYY) and glucagon-like-peptide-1 (GLP-1) which reduce appetite and affect the central nervous system. Now, companies have also developed a weight loss device that can mimic the benefits of bariatric surgery which is called as Maestro Rechargeable System. The US Food and Drug Administration (FDA) has approved the Maestro device in the year 2015. The device is programmed to send electrical pulses to the vagus nerve, thereby blocking some of the signals carried between brain and digestive tract. The device is placed near abdomen by incision and two miniature electrodes are hooked around the left and right trunks of the vagus nerve where the oesophagus joins the stomach. The device interrupts signals from these extensions for 12 hours a day by applying electric current which encourages a feeling of fullness. Hormonal control of hunger and satiety has also come into focus from the recent studies. Leptin which is also known as a satiety hormone works by activation of certain neurons in the hypothalamus to produce pro-opiomelanocortin (POMC) which in turn can activate melanocortin-4 receptor (MC4R) in the nearby cells. This can encourage signals sent through the vagus nerve and other circuits that suppress food intake. Thus, mutation in MC4R can lead to childhood obesity. Now the pharmaceutical companies have developed a therapeutic, called ‘setmelanotide’ which can act on hypothalamus to activate MC4R. Recently, FDA has approved the drug to treat obesity due to POMC deficiency. Recent advances in the research on gut-brain axis brings promise to develop new therapeutics to treat disorders of mood, behaviour, hunger, satiety and so on.
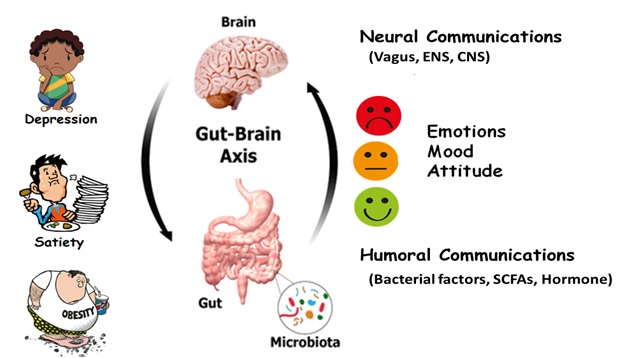
Figure 1: Involvement of gut brain axis in depression, satiety, obesity, mood and behaviour.



