Dr. Purabi Deka Bose
Assistant Professor
Department of Molecular Biology and Biotechnology
Cotton University
Conventional treatment strategies for cancer as existed in last few decades have been surgery, chemotherapy and radiotherapy [1, 2]. Although these therapies have offered substantial benefit for eradication of primary tumors, the incidence of disease relapse from residual malignant cells cannot be ignored.
Our immune system consists of organs, special cells, and substances that help the body to protect from infections and some other diseases. The immune responses are of two types: humoral and cellular, which are mediated by B and T lymphocytes as well as their products [3]. Humoral immunity can neutralize and eradicate extracellular microbes and toxins via antibodies produced by B cells [4, 5, 6], whereas cellular immunity responds more quickly to eradicate intracellular microbes through recognition of antigens, activation of antigen presenting cells (APCs), activation and proliferation of T cells [3, 7, 8].
The immune system protects us from cancer in some ways. The immune response can destroy non-self or foreign substances, such as pathogens or cancer cells. However, cancer cells often escape the surveillance of the immune system as these are not different enough from normal cells or even if these are recognized by the immune system, the response might be very weak. Cancer immunotherapy has become an important therapy for treating cancer in the last few decades. In immunotherapy, certain parts of a person’s immune system are used to fight against cancer. This can be done with different strategies like, 1. modulating body’s own immune system to work efficiently to attack cancer cells, and 2. administering the man-made immune system components to the body.
Types of cancer immunotherapy
Monoclonal antibodies are modified proteins produced to target a specific part of deregulated signal transduction pathways in cancer or to interfere with immunological processes [9, 10]. FDA has already approved more than a dozen mAbs for the treatment of both solid and hematological malignancies, and also more new mAb clinical trials are now being investigated [10, 11]
T cells have the capacity to protect the human body from infection by pathogens and also to clear mutant cells through specific recognition by T cell receptors (TCRs). Cancer immunotherapy has been developed based on this basic recognition process to boost the antitumor efficacy of T cells. T cells genetically equipped with chimeric antigen receptors (CARs) have shown remarkable effectiveness in treating some hematological malignancies, although the efficacy of engineered T cells in treating solid tumors is not that much satisfactory. In summary, with the advent of cancer immunotherapy and recent advances of it, curing cancer seems to be a real possibility for cancer patients.
Cancer vaccines are preventive vaccines and therapeutic vaccines [12]. Preventive vaccines are based on antigens carried by infectious agents associated with carcinogenesis. FDA has approved hepatitis B virus (HBV) and human papilloma virus (HPV) vaccines [12, 13, 14]. Therapeutic vaccines first directly target the immune system and expand the immune system’s attack on cancer cells.
Recent studies have shown that supramolecular peptide assembly (SPA) is a type of nanomaterial with high epitope valency that mimic natural viruses. It has tremendous potential as vaccines (15).
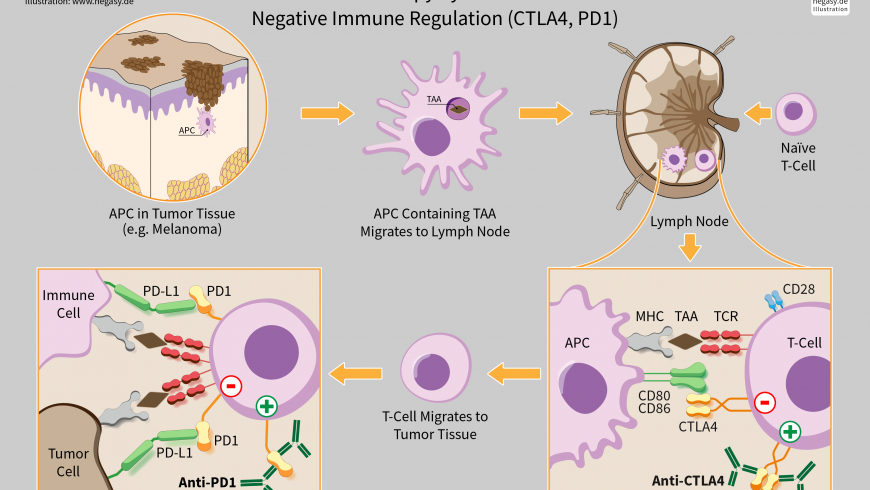
Checkpoints are regulators of the immune system. Some cancer cells can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint molecules are of two types, stimulatory and inhibitory. Stimulatory checkpoint molecules belong to the tumor necrosis factor (TNF) receptor superfamily – CD27, CD40, OX40, GITR and CD137 and B7-CD28 superfamily – CD28 itself and ICOS. Inhibitory checkpoint molecules include A2AR, B7H3, CTL A4, KIR, PD1 etc. Inhibitory checkpoint molecules are targets for cancer immunotherapy due to their potential for use in multiple types of cancers. Currently approved checkpoint inhibitors block CTLA4 and PD-1 and PD-L1. The discovery of immune checkpoints such as CTLA-4 and PD-1 has been crucial to the development of cancer immunotherapy. Drugs or drug candidates that inhibit/block the inhibitory checkpoint molecules are sometimes known as checkpoint inhibitors. The single-agent anti-CTLA-4 and anti-PD-1 have become very effective anticancer treatments in humans. The drug targets of CTLA-4 and PD-1 received FDA approval in the year 2011 and 2014, respectively. Thus, a revolutionary cancer immunotherapy has emerged from recent research and it showed for the first time in many years of research that in metastatic melanoma, there is an improvement in overall survival, with a long term benefit of the patients. For the related basic science discoveries, James P. Allison and Tasuku Honjo won the Tang Prize in Biopharmaceutical Science and the Nobel Prize in Physiology or Medicine in 2018.
Thus, the advent of cancer immunotherapy has made curing cancer a real possibility now. The development of cancer vaccines, CAR-T cell and checkpoint inhibitors has revolutionized the cancer treatment. Recognition and management of toxicities of cancer immunotherapy is also a key factor for the success of cancer treatment. Individualized combination therapies that specifically drive the cancer biology of each patient via new techniques will be the most promising strategies for cancer treatment.
References
- Rius, M. and Lyko, F. (2012). Epigenetic cancer therapy: rationales, targets and drugs. Oncogene. 31: 4257-65.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A. and Ribas, A. (2017). Primary, adaptive and acquired resistance to cancer immunotherapy. Cell. 168: 707-23.
- Bielinska, A.U.; Makidon, P.E.; Janczak, K.W.; Blanco, L.P.; Swanson, B.; Smith, D.M., et al. (2014). Distinct pathways of humoral and cellular immunity induced with the mucosal administration of a nanoemulsion adjuvant. Journal of Immunology (Baltimore, Md: 1950). 192: 2722-33.
- Tsiantoulas, D.; Diehl, C.J.; Witztum, J.L. and Binder, C.J. (2014). B cells and humoral immunity in atherosclerosis. Circulation Research. 114: 1743-56.
- Berek, C. (2016). Eosinophils: important players in humoral immunity. Clinical and Experimental Immunology. 183: 57-64.
- Tan, T.T. and Coussens, L.M. (2007). Humoral immunity, inflammation and cancer. Current Opinion in Immunology. 19: 209-16.
- Ponte, J.F.; Ponath, P.; Gulati, R.; Slavonic, M.; Paglia, M.; O’Shea, A., et al. Enhancement of humoral and cellular immunity with an anti-glucocorticoid -induced tumour necrosis factor receptor monoclonal antibody. Immunology. 130: 231-42.
- Knutson, K.L. and Disis, M.L. (2005). Augmenting T helper cell immunity in cancer. Current drug targets Immune, endocrine and metabolic disorders. 5(4): 365-71.
- Shore, N.D. (2015). Advances in the understanding of cancer immunotherapy. BJU International. 116: 321-9.
- Henricks, L.M.; Schellens, J.H.; Huitema, A.D. and Beijnen, J.H. (2015). The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treatment Reviews. 41: 859-67
- Henricks, L.M.; Schellens, J.H.; Huitema, A.D. and Beijnen, J.H. (2015). The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treatment Reviews. 41: 859-67.
- Maleki, L.A.; Baradaran, B.; Majidi, J.; Mohammadian, M. and Shahneh, F.Z. (2013). Future prospects of monoclonal antibodies as magic bullets in immunotherapy. Human Antibodies. 22: 9-13.
- Speiser, D.E. and Flatz, L. (2014). Cancer immunotherapy drives implementation science in oncology. Human Vaccines & Immunotherapeutics. 10(11): 3107-10.
- Knutson, K.L. and Mittendorf, E.A. (2015). Cancer vaccines in the new era of cancer immunotherapy. Vaccine. 33: 7376.
- Cai, Y.; Ran, W.; Zhai, Y.; Wang, J.; Zheng, C.; Li, Y. and Zhang, P. (2019). Recent progress in supramolecular peptide assemblies as virus mimics for cancer immunotherapy. Biomater Sci. doi: 10.1039/c9bm01380f (Epub: ahead of print).