Sangeeta Das1, Pankaj Deka2 and Anu Malik3
1Ph.D. Scholar, Department of Microbiology, LUVAS, Hisar, Haryana-125001, sangitakashyap9864@gmail.com
2Assistant Professor, Department of Microbiology, CVSc, AAU, Khanapara, Guwahati-781022, drpankajaau@gmail.com
3Ph.D. Scholar, Department of Microbiology, LUVAS, Hisar, Haryana-125001, malikanu1303@gmail.com
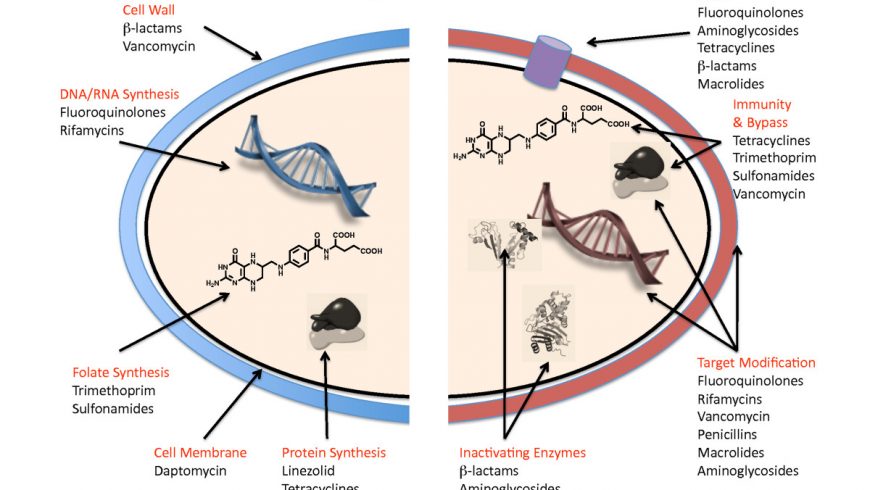
Antimicrobial resistance (AMR) is an urgent worldwide concern and antimicrobial usage in livestock production including poultry, aquaculture and crop farming are among its contributing sources. Antimicrobials are really needed to be used in farms; but due to intensification of farming practices, a large diversity of antimicrobials are often used to raise the poultry flock and livestock with the aim not only to prevent and treat diseases, but also to enhance growth and productivity. However, what is worse the presence of anti-microbial residues in meat and eggs. A large number of such antimicrobials are considered to be of critical importance for human medicine. A recent report estimates that more than 70% of the antibiotics manufactured are used in the agricultural settings. Hence, indiscriminate use of antimicrobials in animal farming system may result in the development of resistant bacteria/ genes (including zoonotic bacteria) finding their way from animals to humans.
Despite the powerful arsenal of weapons against bacteria, the battle against them has not been won due to the menace of AMR. As the bad bugs can nibble back, they need newer drugs. Amongst various means being looked into for combating AMR, microbial predators, including bacteriophages and predatory bacteria, such as Bdellovibrio bacteriovorus, which have been established a century ago, are again in the process of rejuvenation.
It was from Beijerinck’s old idea of a fluid form of life or Pasteur’s idea of fighting microbes that heralded the dawn of phages. Bacteriophages were discovered by Twort (1915) as unidentified molecules that inhibit bacterial growth, but in 1917 D’Herelle was the first to isolate and characterize phages. In an era of increasing emergence of diseases associated with resistant microbes and superbugs, phages are the closest medications we have to serve as a “magic bullet”. The progressive increase in the number of multi-drug resistant bacteria and a paucity of new antibiotics have opened doors for bacteriophages as an alternative means of eliminating pathogens. They are obligate intracellular predators found ubiquitously in the environment wherever susceptible bacteria are available. The surface of the host bacterium possesses specific receptor for phage attachment, thereby, making the host range of each phage quite small. The phage hijacks the cellular machinery of the prey bacterium after injection of the viral genome, and redirects to synthesize and assemble new phage virions. Phages are used as antimicrobial therapies. The first phage therapy was developed by D’Herelle against fowl typhoid induced by Salmonella Gallinarumin chickens. Phage therapy was successfully implemented against open wounds with streptococcal or staphylococcal infections among Soviet soldiers during the Winter War between the former Soviet Union and Finland (1939-40). A therapeutic trial under the program “PhagoBurn” was started by the European Commission in 2013 to treat Pseudomonas aeruginosa infected burn wounds with Good Manufacturing Practices (GMP). Another most striking and well-documented case of phage therapy was reported in 2017, when Top Patterson, an HIV researcher at University of California, San Diego infected with multidrug resistant Acinetobacter baumannii recovered after being in coma for three months using these naturally evolved viruses that attack superbugs. In most of the developed countries, phage therapy has been previously used against infectious diseases in livestock farming and aquaculture. The developing countries are also keeping pace with the usage of phages displaying potential in preventing infectious diseases. However, rapid development of bacterial resistance to bacteriophages through receptor gene mutations, altered nutrient availability, multiple bacterial species, and other means are leading to requirement for phage cocktails for effective therapeutic purposes.
Unlike bacteriophage, the predatory bacteria have recently been the subject of a number of studies to reveal their potential therapeutic uses as an alternative to antibacterial therapies. Predation at the lowest trophic level (i.e. between bacteria) is poorly understood. They are of interest due to their broad Gram-negative bacterial prey range, including a very wide range of human pathogens and lack of simple receptor gene mechanisms for resistance. However, they do not predate on Gram-positive bacterial strains. They invade the prey bacterium by interacting with the outer membrane and creating a pore in the outer membrane and wall, through which they enter into the prey cytoplasm, seal the pore and form a rounded structure called “bdelloplast”. The periplasmic Bdellovibrio secretes many enzymes into the prey cell cytoplasm. The Bdellovibrio utilizes the enzymes and cytoplasmic contents for growth. They divide into multiple progeny cells and grow intracellularly within the enclosed bdelloplast without having to compete with other bacteria for nutrients and are released after lysis. Bdellovibrio invasion results in rapid death of the prey cell. However, synthesis of a paracrystalline S-layer by prey cells may overcome predation. Moreover, predation resistance is not specific for Bdellovibrio but in vitro a small population exhibits a “plastic” resistance phenotype that disappears upon removal of the predator.
Although the two microbial predators act independently, a combination of both might eradicate superbugs under conditions when each alone cannot. This may be also one of the approaches to tackle phage resistance in phage therapy. With the advent of AMR becoming a real global health threat, such combinatorial predator therapy could be considered as a future alternative to antimicrobial therapies. Interestingly, a new study by an international team co-led by Dr. Gerhard Koenig, University of Portsmouth have made another giant leap in fighting AMR by using supercomputers to redesign existing antibiotics which bacteria have outwitted.
References:
- Lambert C, Chang CY, Capeness MJ, Sockett RE. 2010. The first bite: profiling the predatosome in the bacterial pathogen Bdellovibrio. PLoS One 5: e8599. doi:10.1371/journal.pone.0008599
- Kadouri DE, To K, Shanks RMQ, Doi Y. 2013. Predatory bacteria: a potential ally against multidrug-resistant Gram-negative pathogens. PLoS One 8: e63397.
doi:10.1371/journal.pone.0063397 - Oechslin F, Oechslin F. 2018. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses, 10:351. doi:10.3390/v10070351
- Allen HK, Trachsel J, Looft T, Casey TA. 2014. Finding alternatives to antibiotics. N Y Acad. Sci. 1323:91–100. doi:10.1111/nyas.12468
- Antibiotic resistance outwitted by supercomputers. https://www.newswise.com/articles/antibiotic-resistance-outwitted-by-supercomputers
- Atterbury RJ, Hobley L, Till R, Lambert C, Capeness MJ, Lerner TR, Fenton AK, Barrow P, Sockett RE. 2011. Effects of orally administered Bdellovibriobacteriovorus on the well-being and Salmonella colonization of young chicks. Appl Environ Microbiol. 77:5794–5803. doi:10.1128/AEM.00426-11
- Duckworth D. H. (1976). “Who discovered bacteriophage?”. Bacteriological reviews, 40(4), 793–802. https://doi.org/10.1128/br.40.4.793-802.1976
- hemesh Y, Jurkevitch E. 2004. Plastic phenotypic resistance to predation by Bdellovibrio and like organisms in bacterial prey. Environ Microbiol. 6:12–18. doi:10.1046/j.1462-2920.2003.00530. x.
- Kakasis A, Panitsa G. 2018. Bacteriophage therapy as an alternative treatment for human infections: a comprehensive review. J. Antimicrob. Agents.
doi: 10.1016/j.ijantimicag.2018.09.004. - Koskella B, Meaden S. 2013. Understanding bacteriophage specificity in natural microbial communities. Viruses5:806–823. doi:10.3390/v5030806.
- Negus D, Moore C, Baker M, Raghunathan D, Tyson J, Sockett RE. 2017. Predator versus pathogen: how does predatory Bdellovibriobacteriovorus interface with the challenges of killing Gram-negative pathogens in a host setting?Annu Rev Microbiol71:441–457. doi:10.1146/annurev-micro-090816-093618.
- Novel Phage Therapy Saves Patient with Multidrug-Resistant Bacterial Infection. https://ucsdnews.ucsd.edu/pressrelease/novel_phage_therapy_saves_patient_with_multidrug_resistant_bacterial_infect
- Rana, M. S., Lee, S. Y., Kang, H. J., &Hur, S. J. (2019). Reducing Veterinary Drug Residues in Animal Products: A Review. Food Science of Animal Resources, 39(5), 687–703. https://doi.org/10.5851/kosfa.2019.e65
- Reig, M., &Toldrá, F. (2008). Veterinary drug residues in meat: Concerns and rapid methods for detection. Meat science, 78(1-2), 60–67. https://doi.org/10.1016/j.meatsci.2007.07.029
- Sockett RE. 2009. Predatory lifestyle of Bdellovibriobacteriovorus. Rev. Microbiol. 63:523–539. doi: 10.1146/annurev.micro.091208.073346.
- Wernicki, A., Nowaczek, A., & Urban-Chmiel, R. (2017). Bacteriophage therapy to combat bacterial infections in poultry. Virology Journal, 14(1), 179. https://doi.org/10.1186/s12985-017-0849-7
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 5th Revision. (2017). Available from: http://www.who.int/foodsafety/publications/antimicrobials-fifth/en/