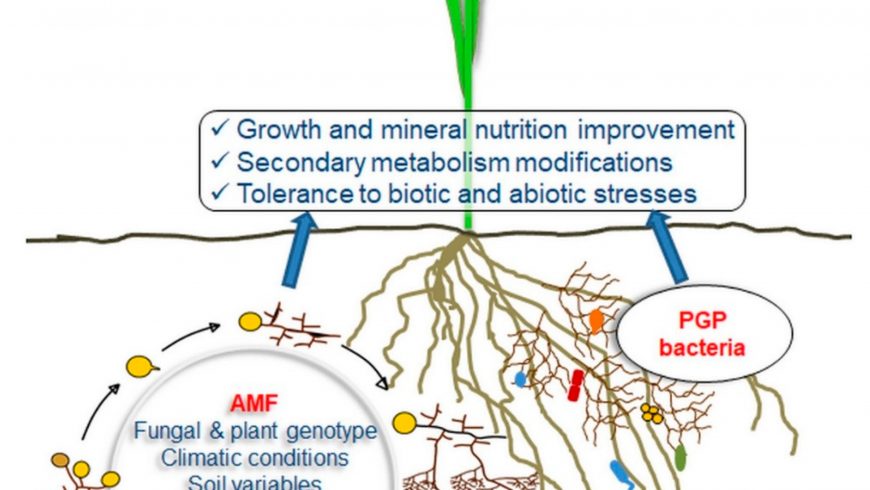
In the face of changing global climate, plants are often subjected to extended durations of abiotic stress. Under suchcircumstances, plants naturally release ethylene, a pivotal signalling molecule that protects against harsh conditions. However, when plants experience consistent and intense stress, they might produce ethylene at unsustainable levels, leading to stunted growth and diminished crop yields. This is where plant growth-promoting bacteria (PGPB) producing 1-aminocyclopropane-1-carboxylate deaminase (ACCD) come into play. By introducing these beneficial bacteria, numerous plants have managed to mitigate the detrimental impacts of excess ethylene, ensuring they thrive even in demanding environments.
Abiotic stress- impact on plant growth and induction of ethylene production
Ethylene is commonly associated with the processes like fruit ripening, flower blooming, and leaf ageing, spikes during periods of abiotic stress in plants. Elevated ethylene levels from stresses such as flooding, droughts, salinity, and metal toxicity, can cause stunted root growth, expedite leaf ageing, and disrupt chlorophyll pigments. Ethylene emerges from a multi-step process; its origin is traced back to methionine. This amino acid undergoes transformation facilitated by SAM synthetase, giving rise to S-adenosyl-l-methionine (SAM). The subsequentchange unfolds with SAM evolving into 1-aminocyclopropane-1-carboxylic acid (ACC) under the guidance of ACC synthetase. In the conclusive phase, ACC is steered toward ethylene conversion by ACC oxidase (ACO). This pivotal conversion necessitates the presence of oxygen and CO2 as indispensable co-substrate and activators. Yet, this intricate synthesis produces a byproduct known as cyanide, a potentially harmful substance. To address this, cyanide is typically extracted from the cell and integrated into L-cysteine, forming the compound β-cyanolalanine, a process orchestrated by β-cyanolalanine synthase (CAS) and possibly bolstered by ethylene and metabolized further.
ACC can sometimes deviate from its standard path to ethylene to produce specific derivative compounds. N-malonylACC (MACC) is a prominent derivative, which typically doesn’t revert to ethylene. In contrast, 1-(γ-L-glutamylamino)ACC (GACC) occurs in lesser quantities. As such, ethylene is a critical influencer in numerous plant growth and response mechanisms, including seed sprouting, ageing processes, stress responses, and extension of roots. When exposed to environmental stresses, plants adjust their ethylene levels to ignite protective signalling pathways. Yet, in intense stress, ethylene concentrations in plant tissues might surpass optimal levels, potentially stalling root and shoot expansion and accelerating leaf ageing, ultimately impacting the plant’s overall vitality and growth.
To maintain crop yield in stressful environments, managing and reducing the ethylene levels induced by stress is vital. Among various approaches explored, enhancing the application of plant growth-promoting bacteria (PGPB) equipped with ACC deaminase (ACCD) activity has emerged as a promising solution. This is because ACCD, present in PGPB, effectively disassembles the ethylene precursor, ACC, turning it into α-ketobutyrate and ammonia. This transformation leads to lowered ethylene concentration in plants, thereby mitigating its negative influence on growth. Furthermore, PGPB improves plant resilience and growth during stressby forming varied interactions, whether rhizospheric, endophytic, or symbiotic. Recent studies have unearthed numerous ACCD-rich bacterial strains across diverse plant species and rhizosphere regions, emphasizing their widespread presence and diverse efficacy levels when introduced to different crops.
Role of PGPB producing ACCD
ACCD, first discovered in a Pseudomonas sp., has been noted for its role in managing ethylene levels in plants by transforming ACC into α-ketobutyrate and ammonia. Impressively, this enzyme is found across the three primary domains of life: archaea, bacteria and eukarya. The enzyme’s activation is multifaceted, influenced by numerous determinants such as temperature and pH levels. As an inducible enzyme, ACCD necessitates a certain threshold of its substrate ACC, with a minimum concentration of 100 nM, essential for Pseudomonas sp. ACCD’s efficacy in degrading ACC is closely associatedto its activity. For instance, with elevated activity, ACCD can influence stress-induced ethylene in various plants. On the other hand, reduced activity might only benefit select plant types or may not be adequate in mitigating excessive ethylene. By measuring α-ketobutyrate concentrations, one can discern varying degrees of ACCD performance among different bacterial strains. Additionally, the mutualistic relationship between specific bacterial strains and their corresponding host plants is pivotal in shaping plant growth.
Selecting bacterial strains with optimal ACCD activity is crucial for the host plant species. Over the last decade, various PGPB strains, each with varying ACCD activities, have been introduced to diverse plants. This has been done to enhance plant growth and yield, especially in environments affected by stress factors like flooding, drought, salinity, and extreme temperatures. The acdS gene, responsible for encoding ACCD activity, is prevalent across numerous bacterial strains. The gene’s expression is influenced by factors such as oxygen availability, the presence of its substrate ACC, regulatory proteins like the leucine responsive regulatory protein (LRP) and nitrogen-fixing regulator (NifA), and ACCD regulatory genes like acdR. However, these factors can vary based on the specific bacterial strain.
Conclusion and future perspectives
As global climate change intensifies, the repercussions of abiotic stresses like flooding, heat, salinity, and drought are magnified. Field-grown plants often grapple with multiple stressors simultaneously. With the world’s population rising, global food security concernsincrease. Given the proven benefits of PGPBs in terms of crop yields amidst these challenges and their environmental advantages, there’s a compelling reason to integrate them more extensively in agriculture. Doing so could curtail reliance on chemical fertilizers and pesticides, cutting cultivation expenses and preserving ecosystems. However, the relationship dynamics between PGPBs and host plants, the ACCD activity levels within PGPBs, and the combined effects of PGPB strains play pivotal roles in influencing crop outcomes under stress. Identifying and employing PGPBs that boast high ACCD activity, harmonizing with specific host plants, and offering collaborative benefits are essential. This process entails comprehensive steps, from isolating diverse bacterial strains to scrutinizing their synergy with designated host plants.
References
Penrose, D.M. and Glick, B.R. (2003). Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 118 (1): 10-15. https://doi.org/10.1034/j.1399-3054.2003.00086.x.
Glick, B.R. (2007). Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci.26(5-6): 227-242. https://doi.org/10.1080/07352680701572966
Singh, R.P.; Shelke, G.M.; Kumar, A. and Jha, P.N. (2015). Biochemistry and genetics of ACC deaminase: a weapon to “stress ethylene” produced in plants. Front. Microbiol.6: 637. https://doi.org/10.3389/fmicb.2015.00937
Naing, A.H.; Maung, T.T.; Kim, C.K. (2021). The ACC deaminase-producing plant growth-promoting bacteria: Influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress.Physiol. Plant.,173(4):1992-2012. https://doi.org/10.1111/ppl.13545
Author Information:
- Name of the Author: Pranami Bharadwaj
- Designation of Author: DST INSPIRE Fellow
- E-mail ID of Author: pranami.bharadwaj@gmail.com
- Name and address of the Institute to which the Authoris associated: Microbial Biotechnology Lab, Institute of Advanced Study in Science and Technology, Guwahati