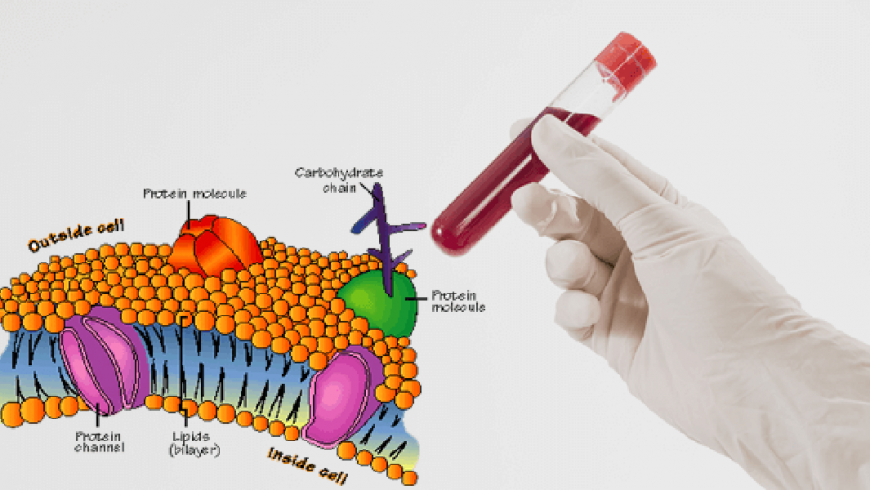
Signal transduction is initiated by complex protein–protein interactions between ligands, receptors and kinases. It is now becoming clear that lipid microenvironments on the cell surface known as lipid rafts also take part in this process. Lipid rafts containing a given set of proteins can change their size and composition in response to intra- or extra-cellular stimuli. This favours specific protein-protein interactions, resulting in the activation of signaling cascades. The Singer–Nicholson fluid mosaic concept is still the textbook model of how the cell membrane is organized. It proposes that the lipid bilayer functions as a neutral two-dimensional solvent, having little influence on membrane protein function. But biophysicists find that lipids exist in several phases in model lipid bilayers, including gel, liquid-ordered and liquid-disordered states, in order of increasing fluidity. In the gel state, lipids are semi-frozen, whereas at the other extreme, in the liquid-disordered state, the whole lipid bilayer is fluid, as proposed by the Singer–Nicholson model. In the liquid ordered phase, phospholipids with saturated hydrocarbon chains pack tightly with cholesterol but nevertheless remain mobile in the plane of the membrane. Despite a detailed biophysical characterization of model membranes, it has been difficult to show that lipids exist in these different phases in the complex environment of the cell.
Membrane lipid dynamics
Lipid molecules are the essential building blocks of cells and are extremely diverse. Theoretically, there are ~180,000 different lipid species that belong to eight different cate gories: glycerol-phospholipids, sterol lipids, sphingolipids, fatty acyls, glycerolipids, prenol lipids, saccharo-lipids and polyketides (Brugger et al., 2014). In mammalian cells, the major lipid species are glycerol-phospholipids, sphingolipids and sterol lipids. Different lipids are co-regulated in cells and this co regulation is organized in a conserved circular network (Koberlin et al., 2015). In particular, lipids that are found in similar metabolic pathways and cellular locations show strong co regulation. Glycero-phospholipids are the most abundant lipid species. They share the common glycerol backbone and all contain a phosphate group (Coskun et al., 2011). The head group of a glycerol-phospholipid can be modified by the addition of various chemical moieties onto the sn 3 position of the glycerol backbone, leading to a number of differ ent phosphatidyl lipids, such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phos phatidylglycerol, phosphatidylinositol or the unmod ified phosphatidic acid. The fatty acid chains in the sn 1 and sn 2 positions of the glycerol backbone can be variable in terms of length and double bonds (number and position), and the linkage to the glycerol backbone can also be varied by ester, alkyl ether, or alkenyl ether bonds. Most glycerol-phospholipids have zwitterionic headgroups, while some minor species have acidic headgroups that carry negative charges.
Lipid distribution: The distributions of membrane lipids are heterogeneous within the plasma membrane. First, the lipid compositions of the inner leaflet and the outer leaflet have substantial differences. For example, acidic glycerol-phospholipids and zwitterionic phosphatidyl ethanolamine are mainly distributed in the inner leaflet, whereas phosphatidylcholine and sphingolipids are mainly in the outer leaflet (Fadeel et al., 2009). Second, the lateral lipid distribution is highly heterogeneous, and the plasma membrane is divided into various nanodomains that pro vide platforms to support local signaling. Acidic glycerol-phospholipids can form nanodomains with a high density of negative charges (van den Bogaart et al., 2009). Through transbilayer lipid–lipid interactions, the clustering of acidic glycerol-phospholipids in the inner leaflet may also regulate the functional lipid domains in the outer leaflet (Raghupathy et al., 2015). It is not fully understood how the heterogeneous distribution of lipids occurs in the plasma membrane. Three types of lipid transporters are involved in regulat ing the vertical lipid asymmetry across the membrane bilayer. Flippase translocates certain lipids from the outer leaflet to the inner leaflet, whereas floppase translocates certain lipids in the opposite direction. Both flippase and floppase require ATP for lipid transloca tion. Scramblase translocates some lipids bidirectionally in an ATP-independent but Ca2+-dependent manner. The mechanisms that govern the lateral heterogeneity of membrane lipids are more complicated. Several models have been proposed to explain the formation of choles terol-rich domains, but the physiological relevance of these models is not yet clear. Recently, the mechanisms controlling the clustering of acidic glycerol-phospholipids have started to be unveiled. Many factors contribute to the clustering process. Ionic interactions between acidic glycerol-phospholipids and membrane proteins can glue these molecules together to form functional domains (van den Bogaart et al., 2009). Moreover, ionic interactions between acidic glycerol-phospholipids and cytoskeleton proteins help the formation and immobilization of lipid domains (Raghupathy et al., 2015). Membrane potential can also affect acidic glycerol-phospholipid clustering (Zhou et al., 2015).
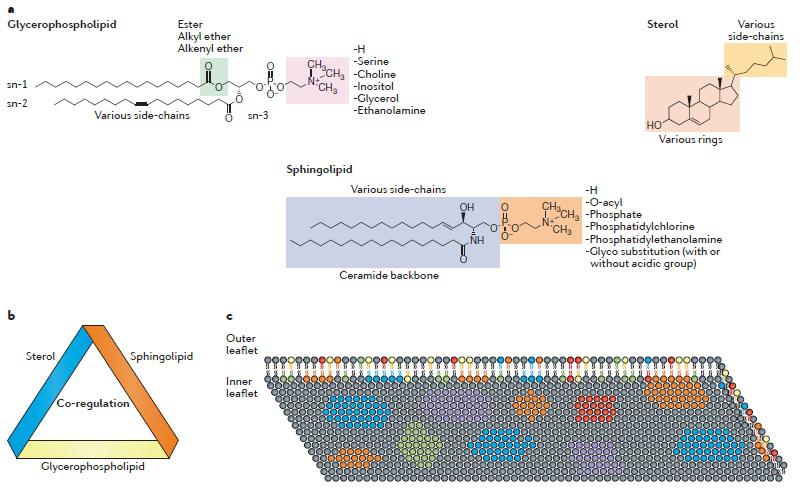
![]()
(Photo courtesy: Wei et al., 2016)
Figure 1. Nature of plasma membrane lipids
Lipid rafts
A turning point came when the lipid raft hypothesis was formulated more than fifteen years ago (Brown et al., 1998, Sankaram et al., 1990). It originated from studies on epithelial cell polarity, and its central postulate was the existence of lipid rafts, consisting of dynamic assemblies of cholesterol and sphingolipids, in the exoplasmic leaflate of the bilayer. The preponderance of saturated hydrocarbon chains in cell sphingolipids allows for cholesterol to be tightly intercalated. The mechanism of inner leaflet coupled to the outer leaflet is not clear. One possibility is that long fatty acids of sphingolipids in the outer leaflet couple the exoplasmic and cytoplasmic leaflets by interdigitation. Transmembrane proteins could also stabilize this coupling. The membrane surrounding lipid rafts is more fluid, as it consists mostly of phospholipids with unsaturated, and therefore kinked, fatty acyl chains and cholesterol. In other words, lipid rafts form distinct liquid-ordered phases in the lipid bilayer, dispersed in a liquid-disordered matrix of unsaturated glycerolipids (Schroeder et al., 2004). The raft concept has long been controversial, largely because it has been difficult to prove definitively that rafts exist in living cells. But recent studies with improved methodology have dispelled most of these doubts.
Raft distribution and trafficking
The distribution of lipid rafts over the cell surface depends on the cell type. In polarized epithelial cells and neurons, lipid rafts accumulate in the apical and axonal plasma membrane, respectively. Basolateral and Somatodenritic membranes also contain rafts, but in smaller amounts. Interestingly, caveolae are present mainly on the basolateral side of epithelial cells, which faces the blood supply and is more active during signal transduction. In lymphocytes and fibroblasts, rafts are distributed over the cell surface without obvious polarity. We can roughly estimate the fraction of the cell surface covered by rafts by comparing the ratio of the main raft and non-raft exoplasmic leaflet lipids, sphingolipids and phosphatidylcholine, respectively. Typically, sphingolipids make up about 45% of the cell surface in fibroblasts (Renkonen et al., 1971) and roughly 30% in lymphocytes (Levis et al., 1976), but these values are upper limits and may also be cell-type dependent. Raft lipids are most abundant at the plasma membrane, but can also be found in the biosynthetic and endocytic pathways. Whereas cholesterol is synthesized in the endoplasmic reticulum (ER), sphingolipid synthesis and head-group modification are completed largely in the Golgi (van Meer et al., 1989). As these data predict, cholesterol–sphingolipid rafts first assemble in the Golgi apparatus. Movement of lipid rafts out of the Golgi seems to be mainly towards the plasma membrane, as vesicles going back to the ER contain little sphingomyelin and cholesterol. The inclusion of proteins into rafts is important for polarized delivery to the cell surface in many cell types (Keller et al., 1997; Ledesma et al., 1997). Lipid raft trafficking does not end with surface delivery rafts are continuously endocytosed from the plasma membrane (Mukherjee et al., 2000). From early endosomes, rafts either recycle directly back to the cell surface or return indirectly through recycling endosomes, which could also deliver rafts to the Golgi (Puri et al., 1999).
Rafts in signal transduction:
The most important role of rafts at the cell surface may be their function in signal transduction. It is well established that, in the case of tyrosine kinase signaling, adaptors, scaffolds and enzymes are recruited to the cytoplasmic side of the plasma membrane as a result of ligand activation (Hunter et al., 2000). One way to consider rafts is that they form concentrating platforms for individual receptors, activated by ligand binding. If receptor activation takes place in a lipid raft, the signaling complex is protected from non-raft enzymes such as membrane phosphatases that otherwise could affect the signaling process. In general, raft binding recruits proteins to a new micro-environment, where the phosphorylation state can be modified by local kinases and phosphatases, resulting in downstream signaling.
Immunoglobulin E signaling:
The first signaling process convincingly shown to involve lipid rafts was immunoglobulin E (IgE) signaling during the allergic immune response (Field et al., 1995).This signaling pathway is activated when IgE binds through its Fc segment to receptors (FcεRI) residing in the plasma membrane of mast cells and basophiles. FcεRI is monomeric and binds one IgE molecule. The receptor is activated by the binding of oligomeric antigens to receptor-bound IgE. Cross-linking of FcεRI by oligomeric antigens activates the transmembrane signaling process, ultimately leading to release of the chemical mediators of allergic reactions. The Fc receptor is a tetramer composed of one α-, one β- and two γ-chains (Baird et al., 1999). The α-chain binds IgE and the β- and the γ-chains contain immune receptor tyrosine based activation motifs (ITAMs), common to all multi-subunit immune recognition receptors. Cross-linking of two or more of these receptors by antigens recruits the doubly acylated non-receptor Src-like tyrosine kinase Lyn, which is thought to initiate the signaling cascade by phosphorylating ITAMs so that they can bind to Syk/ZAP-70 family tyrosine kinases through their phosphotyrosine residues. Syk is activated by phosphorylation and this, in turn, leads to activation of phospholipase Cγ (PLCγ). Finally, downstream signaling results in increased calcium levels in the proximity of the membrane, and this triggers the release of histamine from nearby granules.
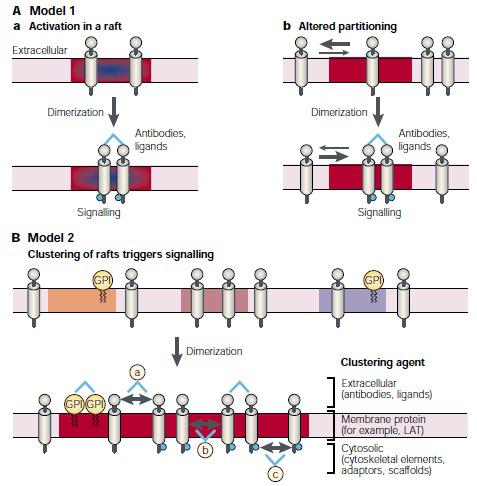
(Photo courtesy: Kai et al., 2000)
Figure 2: Models of how signaling could be initiated through raft.
T-cell antigen receptor signaling:
The T-cell antigen receptor (TCR) is another multi-subunit immune recognition receptor that engages lipid rafts during signaling. The TCR is composed of αβ-heterodimers which associate with the CD3 (γδε) complex and the ζ-homodimer. Whereas the α- and β-subunits contain the extracellular binding site for peptides that are presented by the MHC class I and II proteins on the surface of APCs, the CD3 and ζ-subunits contain cytoplasmic ITAM motifs. The earliest signaling event after TCR engagement is the phosphorylation of ITAM tyrosine residues by the doubly acylated non-receptor Src-like tyrosine kinases, Lyn and Fyn (Janes et al., 2000). When ZAP-70 binds to phosphorylated ITAMs, it is activated and in turn, phosphorylates LAT, a transmembrane protein that couples TCR activation to several signaling pathways (Langlet et al., 2000). Several GPI-linked proteins and accessory molecules help to amplify the T-cell activation events. Phosphatases are also required to switch these pathways on and off (Carry et al., 2000).
GDNF signaling:
The glial-cell-derived neurotrophic factor (GDNF) family of ligands is important for the development and maintenance of the nervous system. In addition, they function during differentiation of the kidney and spermatogonia. GDNF binds to a multicomponent receptor complex that is composed of the GPI-linked GDNF receptor-α (GFRα) and the transmembrane tyrosine kinase, RET. The receptor subunits GFRα and RET are not associated with each other in the absence of ligand. But after extracellular GDNF stimulation, RET moves into rafts, where it associates with GFRα. Signal transduction depends on the co-localization of RET and GFRα in lipid rafts, as cholesterol depletion with methyl-β-cyclodextrin decreases GDNF signaling (Tansey et al., 2000).
Ras signaling:
The small GTPase Ras is central to many signaling processes. It acts as a switch that, when activated, recruits serine/threonine kinases of the Raf family to the plasma membrane. These, in turn, activate the ERK–MAP kinase pathway and other targets. The two Ras isoforms, K-Ras and H-Ras, are almost identical in sequence but have different signaling properties (Roy et al., 1999). Both isoforms have a carboxy-terminal prenylated CAAX sequence. Whereas K-Ras has a polybasic region required for plasma membrane localization, H-Ras is palmitoylated and therefore more likely to partition into lipid rafts. Expression of a dominant-negative mutant of caveolin strongly inhibited H-Ras-mediated Raf activation, but had no effect on its activation by K-Ras. The expression of this mutant led to a decrease in the number of caveolae on the cell surface, and depleted cell surface cholesterol. The mutant phenotype could be mimicked by depleting cholesterol with methyl-β-cyclodextrin and it could be rescued by addition of exogenous cholesterol. One interpretation of these results is that expression of the caveolin mutant reduces the cholesterol content of the plasma membrane and therefore the number of functional lipid rafts. As H-Ras can signal only through rafts, it can no longer activate Raf. But K-Ras, which does not operate in rafts, is not affected.
Hedgehog signaling:
Drosophila melanogaster Hedgehog and its mammalian homologues act as short-range morphogens during tissue patterning. In the absence of Hedgehog signaling, the sterol-sensing membrane protein patched represses the constitutive signaling activity of a second membrane protein, smoothened by forming an inactive patched smoothened complex (Incardona et al., 2000). Hedgehog binding to patched releases smoothened, which activates a signaling cascade that culminates in the upregulation of a specific set of nuclear transcripts Hedgehog is an interesting signaling molecule, as it is post-translationally modified to introduce a cholesterol moiety at the carboxyl terminus and a palmitate moiety at the amino terminus (Porter et al., 1999). Cholesterol-modified Hedgehog is membrane bound, and has been shown to associate with lipid rafts in Drosophila embryos. The cholesterol modification restricts the signaling range of Hedgehog, making it a short-range morphogen. If Hedgehog is mutated to lose its hydrophobic anchor, it is secreted and can activate cells much further away than normal (Burke et al., 1999). If cholesterol is replaced with a GPI-anchor which should still localize the protein to rafts Hedgehog is no longer released from the surface of the expressing cells (Porter et al., 1999). Another sterol sensing protein, dispatched, is also required for the release of Hedgehog (Porter et al., 1999).The mechanism of release could involve either displacement of the cholesterol tether or shedding of membrane vesicles from Hedgehog producing cells. In conclusion, the requirement for lipid rafts during Hedgehog signaling is completely different from that described for other signaling processes. The cell biology of this fascinating signaling process is poorly understood, and awaits a detailed exploration.
Summary and future directions
The lipids membrane has remarkable functions in cell signaling. Many cell signaling proteins have been found to specifically interact with lipid molecules via distinct modes. Indeed, a single lipid molecule can have multiple regulatory functions. For example, cholesterol can mediate TCR clustering but also serve as gatekeeper to inhibit spontaneous TCR activation. Moreover, cholesterol binding might retard TCR mobil ity in the membrane. However, it is still at a very early stage of fully understanding the physiological and pathological functions of membrane lipids, which has sig nificantly slowed down the development of a new genera tion of medicines.
References:
Baird, B., Sheets, E. D. & Holowka, D. (1999). How does the plasma membrane participate in cellular signaling by receptors for immunoglobulin E? Biophys. Chem. 82, 109–119.
Brown, D. A. & London, E. (1998). Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 14, 111–136.
Brugger, B. (2000). Segregation from COPI–coated vesicles of sphingomyelin and cholesterol. J. Cell Biol.
Brugger, B. (2014). Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu. Rev. Biochem. 83, 79–98.
Burke, R. (1999). Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–815.
Cary, L. A. & Cooper, J. A. (2000). Molecular switches in lipid rafts. Nature 404, 945–947 .
Coskun, U. & Simons, K. (2011). Cell membranes: the lipid perspective. Structure 19, 1543–1548.
Eggeling, C. (2009). Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162.
Fadeel, B. & Xue, D. (2009). The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277.
Field, K. A., Holowka, D. & Baird, B. (1995). Fc epsilon RImediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc. Natl Acad. Sci. USA 92, 9201–9205.
Hunter, T. (2000). Signaling and beyond. Cell 100, 113–127.
Incardona, J. P. & Eaton, S. (2000). Cholesterol in signal transduction. Curr. Opin. Cell Biol. 12, 193–203.
Janes, P. W., Ley, S. C., Magee, A. I. & Kabouridis, P. S. (2000). The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12, 23–34.
Keller, P. & Simons, K. (1997). Post-Golgi biosynthetic trafficking. J. Cell Sci. 110, 3001–3009
Koberlin, M. S. (2015). A conserved circular network of coregulated lipids modulates innate immune responses. Cell 162, 170–183.
Langlet, C., Bernard, A. M., Drevot, P. & He, H. T. (2000). Membrane rafts and signaling by the multichain immune recognition receptors. Curr. Opin. Immunol. 12, 250–255.
Ledesma, M. D., Simons, K. & Dotti, C. G. (1998). Neuronal polarity: essential role of protein–lipid complexes in axonal sorting. Proc. Natl Acad. Sci. USA 95, 3966–3971.
Levis, G. M. & Evangelatos, G. P. (1976). Lipid composition of lymphocyte plasma membrane from pig mesenteric lymph node. Biochem. J. 156, 103–110.
Mukherjee, S. & Maxfield, F. (2000). Role of membrane organization and membrane domains in endocytic lipid trafficking. Traffic 1, 203–211.
Porter, J. A., Young, K. E. & Beachy, P. A. (1996). Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–259.
Puri, V. (1999) Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nature Cell Biol. 1, 386–388.
Raghupathy, R. (2015). Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161, 581–594.
Renkonen, O., Kaarainen, L., Simons, K. & Gahmberg, C. G. (1971). The lipid class composition of Semliki forest virus and plasma membranes of the host cells. Virology 46, 318–326.
Roy, S. (1999). Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nature Cell Biol. 1, 98–105.
Sankaram, M. B. & Thompson, T. E. (1990) Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry 29, 10670–10675.
Schroeder, R., London, E. & Brown, D. (1996). Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl Acad. Sci. USA 91,12130–12134.
Tansey, M. G., Baloh, R. H., Milbrandt, J. & Johnson, E. M. (2000). Jr GFRα-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25, 611–623.
van den Bogaart, G. (2011). Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555.
van Meer, G. (1989). Lipid traffic in animal cells. Annu. Rev. Cell Biol. 5, 247–275 (1989).
Wei, W., Xiaoshan, S. and Chenqi, X. (2016). Regulation of T cell signalling by membrane lipids. Nature 16, 690-701.
Wang, Y. H. (2012). Divalent cation-induced cluster formation by polyphosphoinositides in model membranes. J. Am. Chem. Soc. 134, 3387–3395.
Zhou, Y. (2015). Signal transduction. Membrane potential modulates plasma membrane phospholipid dynamics and K Ras signaling. Science 349, 873–876.
Rupam Dutta Research Associate, Dept. of Animal Biotechnology, College of Veterinary Science, Khanapara, Guwahati-22.