A healthy life is dream for all and a universal goal. Infection is inconvenient; it affects our lifestyle, disrupts the equality of life and shortens the life. Sulfur mustard (Cl-CH2CH2)2S is one of the cytotoxic organic compounds. It is a bifunctional alkyl ting agent which is also called as a Mustard gas or Mustard agent. Sulfur mustard is a cell poison that causes disruption and impairment of a variety of cellular activities that are dependent upon a very specific integral relationship. Sulfur mustard (SM) is a yellow to brown oily liquid with a slight garlic or mustard odor and its vapors can reach hazardous levels during warm weather and it has been used as warfare agent. Mustard agent can easily be dissolved in most organic solvents but has poor solubility in water. Despite the fact that this chemical weapon is called mustard gas, it’s not a gas, but rather a very thick, volatile liquid. The plumes of mustard gas which wafted over the trenches of the First World War were created by aerosolizing the liquid, typically by encasing it in fired projectiles. The primary effect of SM exposure on epithelial cells is cutaneous toxicity, in addition to ocular, respiratory and other tissue injuries. Detailed understanding of the molecular mechanisms involved in HD-induced skin toxicity is important for the development of effective counter-measures and therapeutics against this chemical warfare agent of mass destruction. Several reports show that SM-induced tissue toxicity is mainly due to its alkylating properties, enhanced production of inflammatory cytokines and increased oxidative stress owing to production of damaging reactive oxygen species. SM penetrates into skin tissue, attacks mainly the dividing basal epidermal cells and causes massive inflammatory response, apoptotic and necrotic cell death as well as delayed excruciating vesication. These cytotoxic effects are manifested in widespread metabolic disturbances, variable characteristics of which are observed in enzymatic deficiencies, vesicant action, abnormal mitotic activity and cell division, bone marrow disruption, disturbances in hematopoietic activity, and systemic poisoning. Indeed, mustard gas readily combines with various components of the cell such as amino acids, amines, and proteins. The Sulfur Mustard mono-analog derivative 2-chloroethylethyl sulphide (CEES) has been studied to gain insight into the mechanism of action of SM. CEES is also involved in the induction of cell death by enhancement of the DNA repair process, which activates poly(ADP-ribose) polymerase (PARP) with the subsequent NAD depletion. Acute lung injury is a principal cause of morbidity and mortality in response to mustard gas (SM) inhalation. Glutathione (GSH) is known to have a protective effect against SM toxicity, and detoxification of SM is believed to occur, in part, via GSH conjugation. The proliferating basal epidermal cells are major primary sites of attack during SM-caused skin injury. As compared to the control, dose-dependent decreases in cell viability and proliferation as measured by DNA synthesis, together with S and G2-M phase arrest, are observed in cell cycle progression. The mechanisms regulating expression of these mediators were studied using an in vitro skin construct model in which mouse keratinocytes were grown at an air-liquid interface and exposed directly to 2-chloroethyl ethyl sulfide (CEES), a model sulfur mustard vesicant. CEES were found to cause marked increases in keratinocyte protein carbonyls, a marker of oxidative stress. CEE modulates expression of antioxidants and enzymes producing inflammatory mediators by distinct mechanisms due to increases in antioxidants which may be an adaptive process to limit tissue damage.
Reactive oxygen species (ROS) are produced during normal physiological processes such as energy production, which inevitably leads to the generation of oxidative molecules: superoxide (O2._), hydrogen peroxide (H2O2) or hydroxyl radical (.OH). Many of the ROS-mediated responses actually protect the cells against oxidative stress. On the other hand, over production of ROS has the potential to cause damage. Free radicals are also known as ROS and RNS. This unpaired electron usually gives a considerable degree of reactivity to the free radical. CEES is used to identify DNA damage repair pathways and signaling events that are activated after exposure to the agent. Although there are compound mechanisms implicated in SM-induced inflammation and toxicity, one of the important reported mechanisms is the depletion of glutathione (GSH), which probably leads to an increase in the production of ROS and consequent membrane lipid peroxidation. The exogenous addition of antioxidants such as GSH, N-acetylcysteine (NAC), vitamin E, superoxide dismutase, catalase, sulforaphane, quercetin and catalytic antioxidants such as manganese (III) meso-tetrakis (di-N-ethylmidazole) porphyrin (AEOL 10150) have been reported to attenuate lung and skin injury by SM/CEES. Superoxide dismutation via SOD enzyme is present in all aerobic organisms and in all subcellular compartments susceptible of oxidative stress. Catalase and peroxides enzymes execute the detoxification of ROS. The elimination of excess H2O2 participate in the fine regulation of the H2O2 -concentration in the cell. The initiation phase includes activation of O2 and is rate limiting. Polyunsaturated fatty acids (PUFA, the main components of membrane lipids) are susceptible to peroxidation. Catalytic activation of the nuclear enzyme poly (ADP-ribose) polymerase (PARP-1) has been demonstrated to be a major event in response to high levels of DNA damage, and PARP-1 activation may be part of apoptotic signaling. Therefore, CEES which is commercially available, is a valid alternative instead of SM to perform most in vitro experiments. SM penetrates into skin tissue, attacks mainly the dividing basal epidermal cells causing massive inflammatory response, apoptotic and necrotic cell death, as well as delayed excruciating vesication. Generally in SM, its exposure causes the depletion of cellular antioxidant thiols, mainly GSH, which may lead to an increased production of reactive oxygen species (ROS) and membrane lipid peroxidation. It has been shown that when half mustard gas is induced into the human monocytes THP-1 then it releases TNF-alpha. DNA damaging effect of CEES also induce oxidative stress and oxidative DNA damage in the cell types, as measured by an increase in mitochondrial and cellular reactive oxygen species.
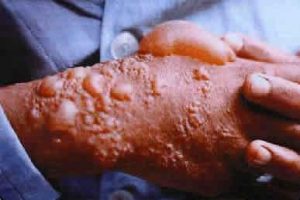 Effect of mustard gas on skin
Effect of mustard gas on skin
Source: http://www.oldmagazinearticles.com/WW1_Mustard_Gas_Warfare_newspaper#.W04EstJKjDc
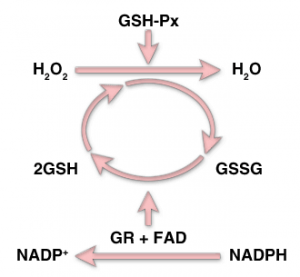
Removal of hydrogen peroxide (H2O2) from the cells and its conversion to H2O by the enzyme glutathione peroxides (GSH-Px)/
Source: http://themedicalbiochemistrypage.org/aminoacidderivatives.php
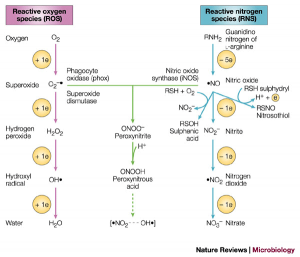
Relationship between ROS and RNS/
Source: http://www.rki-i.com/doc/oxistress.htm
References:
- Tewari-Singh, N.; Rana, S.; Gu, M.; Pal, A.; Orlicky, D.J.; White, C.W. and Agarwal, R. (2009). Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin in SKH-1 hairless mice. Toxicol Sci. 108(1): 194-206.
-
Dacre, J.C. and Goldman, M. (1996). Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol. Rev. 48(2):289-326.
-
Wattana, M. and Bey, T. (2009). Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat. Prehosp Disaster Med. 24(1): 19-29.
-
Mérat, S.; Perez, J.P.; Rüttimann, M.; Bordier, E.; Lienhard, A. Lenoir, B. and Pats, B. (2003). Acute poisoning by chemical warfare agent: sulfur mustard. Ann. Fr. Anesth. Reanim. 22(2): 108-18.
-
Grahame, D.A.; Gencic, S. and DeMoll, E. (2005). A single operon-encoded form of the acetyl-CoA decarbonylase/synthase multienzyme complex responsible for synthesis and cleavageof acetyl-CoA in Methanosarcina thermophila. Arch. Microbiol. 184(1): 32-40.
-
Shakarjian, M.P.; Heck, D.E.; Gray, J.P.; Sinko, P.J.; Gordon, M.K.; Casillas, R.P.; Heindel, N.D.; Gerecke, D.R.; Laskin, D.L. and Laskin, J.D. (2010). Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol. Sci. 114(1): 5-19.
-
Paromov, V.; Suntres, Z.; Smith, M. and Stone, W.L. (2007). Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. J. Burns Wounds. 30 (7): e7.
-
Ghanei, M. and Harandi, A.A. (2010). Lung carcinogenicity of sulfur mustard. Clin. Lung Cancer. 11(1): 13-7.
Jesminwara Begum M.Sc. Biotechnology, KIIT University.