A world without bacteria and other microbes wouldn’t support life as we know it. We and other animals would not be able to digest food without the microscopic creatures, which live in our gut microbiomes. Each of us hosts some 40 trillion bacteria in our own personal biomes – some suggest that we are having 90% bacteria and 10% human. It is estimated that the human microbiome, principally that of the gut, consists of ∼1013–1014 micro-organisms, which is more than ten times the number of cells in the human body. The gut microflora benefits their host by protecting against colonisation by pathogens, assisting in intake of nutrients from the diet, metabolising certain drugs to functional forms, and in the absorption and distribution of fat. Waste water treatment systems would fail, and nothing would be able to decompose so waste would pile up if there is no microbe. Without microbes the nutrient recycling underlying life, as we know it, would cease, oceans would become virtually non-productive and asphyxiation of aerobic life would occur. Besides, bacteria are known to produce major diseases of man, animals and plants. How do these small organisms accomplish such big tasks? Working in groups, bacteria are able to communicate using a cell-to-cell chemical communication process enabling them to show a synchronize behaviour on a population-wide scale and accomplish several tasks.
Unlike Eukaryotes, bacteria communicate with one another using chemical signalling molecules as words. Specifically, they release, detect and respond to the accumulation of these molecules, which are called autoinducers. These autoinducers can be regarded as languages used by the bacteria to communicate. When a bacterium is alone, these molecules simply float away. But when there is a large group of bacteria, these secreted molecules increase in proportion to the number of bacteria emitting them. When the molecules reach a certain amount, the bacteria can tell how many neighbours it has, and suddenly all the bacteria begin to act as a synchronized group. This process of intercellular communication is now called Quorum Sensing (QS). The term quorum comes from Latin, and it became popular in the political and social settings much before it came to be used in science. Its use was based on the rules of a British tribunal, the “Justices of the Quorum”, which required the mandatory presence of at least one of the members of the group in order to reach agreements. This presence was extended to the demand of a numerical majority or qualified minority prior to the start of sessions in order for any conclusions to be considered valid. Once the existence of quorum is accepted, the group behaves differently and there is a social process for developing certain actions. In the same way in bacteria, once a certain number of colonies is reached, they begin to behave “socially” and to defend themselves, attack the host, reproduce and emit a series of signals, etc. Quorum sensing confuses the distinction between prokaryotes and eukaryotes because it allows bacteria to behave as multicellular organisms, and to reap benefits that would be unattainable to them as individuals. Many bacterial behaviours are regulated by quorum sensing, including symbiosis, virulence, antibiotic production and biofilm formation. The capacity to behave collectively as a group has obvious advantages; for example, the ability to migrate to a more suitable environment/better nutrient supply and to adopt new modes of growth, such as sporulation or biofilm formation may afford protection from deleterious environments. Molecular communication allows 600 different species of bacteria to organize themselves into the slimy dental plaque that leads to tooth decay, for example, and it likely enables the nasty pathogens that cause streptococcal pneumonia or bubonic plague to time the release of their toxins for maximum impact on their human hosts.
Many bacteria have been found to regulate diverse physiological processes and group activities through quorum sensing. It has long been understood that in infectious diseases, the invading bacteria need to reach a critical cell density before they express virulence and overwhelm the host defence mechanisms, before they initiate an infectious disease. Pseudomonas aeruginosa, a Gram-negative bacterium, is considered as one of the most common opportunistic pathogens in human infections causing fatal systemic disease under certain conditions. This pathogen is particularly common in patients with cystic fibrosis. It has been shown that the quorum-sensing circuits in P. aeruginosa orchestrate a symphony of several virulence factors, such as exoproteases, siderophores, exotoxins and rhamnolipids (Smith and Iglewski, 2003). Similarly, S. aureus is a leading cause of nosocomial infections worldwide and causes diseases from mild skin infections to potentially fatal systemic disorders. Many infections caused by S. aureus, such as endocarditis, osteomyelitis and foreign-body related infections are not caused by free-living cells but rather by colonization or biofilms. Many virulence factors involved in staphylococcal infections, including surface-associated adhesins, hemolysin, toxins and autolysins, are regulated by quorum sensing via the accessory gene regulator (agr) system (Yarwood et al., 2004).
This process of intercellular communication of bacteria was first described in the bioluminescent marine bacterium Vibrio fischer (Nealson and Hastings, 1979). Prior to 1994, quorum sensing was commonly referred to as “autoinduction” (Fuqua et al., 1994). The term “quorum sensing” was introduced by Dr. Steven Winans in 1994, who was putting together one of the first review articles on autoinduction in bacteria. Recent studies show that highly specific as well as universal quorum sensing languages exist which enable bacteria to communicate within and between species. The discovery of intercellular communication among bacteria has led to the realization that bacteria are capable of coordinating activity, that was once believed to be restricted to multicellular organisms. Through the use of autoinducers, bacteria can regulate their behaviour according to population density. Detection of autoinducers allows bacteria to distinguish between low and high cell population density, and to control gene expression in response to changes in cell number. During the 1970s and ’80s, several research labs began searching for clues as to how cell density controls light production in V. fischeri (Engebrecht et al., 1983). One of the first key discoveries came from JoAnne Engebrecht and Kenneth Nealson, who were conducting experiments in Michael Silverman’s lab. Engebrecht and Nealson identified a 9-kilobase (kb) region of the V. fischeri genome that confers light production in high-cell-density cultures, but not low-cell-density cultures, when it is transferred to another bacterium (Escherichia coli) that is normally unable to produce light under any circumstances.
In the natural environment, there are many different bacteria living together, which use various classes of signalling molecules. In some cases, a single bacterial species can have more than one QS system and therefore use more than one signal molecule. The bacterium may respond to each molecule in a different way. In this sense, the signal molecules can be thought of as words within a language, each having a different meaning. Several types of signalling molecules have been identified in the last two decades. N-acylated-L-homoserine lactones (AHLs) are the most common class of signalling molecules in Gram negative bacteria, while autoinducing peptides (AIPs) are the major class of signalling molecules in Gram positive bacteria. Autoinducer-2 (AI-2) is a third category of signalling molecules that is employed by both Gram positive and Gram negative bacteria and is believed to be used for intra- and inter-species communication. Among QS signalling molecules, the most intensely studied signals are AHLs of Gram negative bacteria. The AHL signal is synthesized at low basal level by an enzyme (a LuxI type synthase). AHL signals diffuse out from bacterial cells and accumulate in the medium as a function of cell growth. At a threshold population density, the accumulated AHLs interact with a transcriptional activator protein (a LuxR type transcriptional regulator), which will then induce the expression of QS-regulated genes (Bosgelmez, 2003). In contrast to those in Gram-negative bacteria, there are two types of quorum-sensing systems identified in Gram-positive bacteria. In the first type, quorum-sensing systems consist of a signalling peptide known as autoinducing peptide (AIP) and a two-component signal transduction system (TCSTS) that specifically detects and responds to an AIP. In contrast to AHL signals, cell membrane is not permeable to AIP but a dedicated oligopeptide transporter, largely an ABC transporter, is required to secrete AIP into the extracellular environment. Most of the signalling peptides in Gram-positive bacteria typically consist of 5–25 amino acids and some contain unusual side chains (Dunny and Leonard, 1997).
Till the last decade, the best characterized form of intercellular bacterial communication was known to be mediated by extracellular signalling molecules (Bassler and Losick, 2006; Ng and Bassler, 2009). This type of communication is, however, constrained by the ability of bacteria to secrete and/or recognize the signal, transduce the received information, and modulate gene expression correspondingly. Researchers have identified a previously uncharacterized type of bacterial communication mediated by nanotubes that bridge neighbouring cells. Using Bacillus subtilis as a model organism, it has been visualized to mediate transfer of cytoplasmic fluorescent molecules between adjacent cells. Additionally, by co-culturing strains harbouring different antibiotic resistance genes, molecular exchange can take place; this enables cells to transiently acquire non-hereditary resistance. Furthermore, non-conjugative plasmids could be transferred from one cell to another, thereby conferring hereditary features to recipient cells. Electron microscopy revealed the existence of variously sized tubular extensions bridging neighbouring cells, serving as a route for exchange of intracellular molecules. These nanotubes are also formed in an interspecies manner, between B. subtilis and Staphylococcus aureus, and even between B. subtilis and the evolutionary distant bacterium Escherichia coli. Communicating by nanotubes enables bacteria a straightforward immediate transfer of information that can cross the inherent species barrier. Moreover, because tunnels presumably maintain inner-cellular physiological conditions, channelled molecules are potentially protected from degrading enzymes and harsh environmental conditions. Taken together, nanotube-mediated informational flow is potentially both continuous and efficient, and can enable molecular transfer across long distances in bacteria grown on solid surfaces (Dubey and Yehuda, 2011).
QS research has many potential applications, most of these involve controlling bacteria by interfering with their signalling systems. For example, many bacteria rely on QS to control the expression of the genes which cause disease. If we can block the QS system we may be able to prevent these bacteria from being dangerous. Specifically, the secretion of enzymes that destroy the autoinducers are used to render quorum sensing bacteria mute and deaf. Analogous synthetic strategies are now being explored for the development of novel antimicrobial therapies. Today, a global concern has emerged that we are facing a post antibiotic era with a serious constraint to fight bacterial infections. The increasing occurrence of multi-antibiotic-resistant pathogenic bacterial strains has gradually made conventional antimicrobial treatment ineffective. Thus, there is a great need to develop novel strategies to combat microbes without selecting for resistance. The discovery of bacterial cell-to-cell communication or quorum sensing (QS) system to regulate the bacterial pathogenicity and virulence factor production makes this system a novel therapeutic target for the design and development of a new class of drugs that potentially control pathogenicity. It has been estimated that bacterial biofilms are responsible for over 80% of human infections. Biofilm-associated infections are very difficult to treat with traditional antibiotics as bacteria are embedded in an extracellular matrix composed of exopolysaccharides and proteins that reduce the contact between bacteria and antibacterial agent (Hoiby et al., 2010). Targeting the QS systems of pathogens is a novel plan of attack. Inhibition of bacterial QS, rather than bactericidal or bacteriostatic strategies, offers a promising way to tackle the bacterial resistance problem. Because QS is not directly involved in processes essential for growth of bacteria, inhibition of QS should not impose harsh selective pressure for the development of resistance as with antibiotics. In addition, compounds that are capable of disrupting bacterial QS could potentially be used in combination with conventional antibiotics to increase the efficiency of disease control and to extend the life span of current antimicrobials. A large number of structurally diverse QS inhibitors have been discovered. Of the numerous molecules able to inhibit QS `in vitro`, a few have been successfully tested `in vivo` in animal models. Future work will reveal the possibility of using these QS inhibitors to treat bacterial infections in animals and humans. Quorum sensing is emerging as an integral component of bacterial global gene regulatory networks responsible for bacterial adaptation in biofilms. Another key challenge is to determine functional consequences of quorum sensing in multi-species biofilms. Future research will clearly address these questions in the emerging field of bacterial social behaviours.
Microbial endocrinology has also been recognised as an area of research, that has its foundation and through their long coexistence with animals and plants, microorganisms have evolved systems for sensing host-associated chemicals such as hormones. These hormone sensors enable the microbe to recognise that they are within the locality of a suitable host and for commensals, that it is the appropriate time to initiate expression of genes involved in host colonisation or in the case of pathogens, genes for virulence determinants (Lyte, 2004). To date, the majority of microbial endocrinology investigations have focused on the interaction of bacteria with stress-associated biochemicals. This came about because of the long-held view that stress in humans and animals increases their risk of developing an infection due to stress hormone reductions in immune function (Reiche et al., 2004). However, viewing host stress through the lens of microbial endocrinology provides a broader appreciation of what may be happening with our billions of microbial inhabitants by also considering the impact of the stress event from the perspective of the microbe. In the context of animal welfare, microbial endocrinology has been shown to provide a useful platform on which to develop a holistic understanding of the factors that shape the interactions between microbes and their host during health and disease (Freestone et al., 2008). Through a process recently termed “telesensing” (Roux et al., 2009), certain bacteria can not only sense their population density via quorum sensing, but also can detect and respond to host-stress derived signals such as cytokines, end-products of ischemia, immune cell environments, etc. that are unique to host tissues exposed to stressful conditions (Sansonetti, 2004).
A good researcher always gets recognition. Dr. Bonnie Bassler, who is the Squibb Professor and Chair of the Department of Molecular Biology at Princeton University and an investigator of the Howard Hughes Medical Institute, studied the universal use of chemical communication among bacteria and proposed that interfering with bacterial cell-to-cell communication could form the basis of new antibacterial therapies. She has been selected for the University of Pittsburgh’s 2018 prestigious Dickson Prize in Medicine. The Dickson Prize in Medicine is the highest honour of the University awarded annually to an American biomedical researcher who has made significant, progressive contributions to medicine. The award consists of a specially commissioned medal, a $50,000 honorarium and travel expenses to Pitt to accept the award.
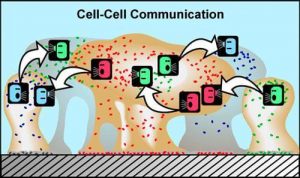
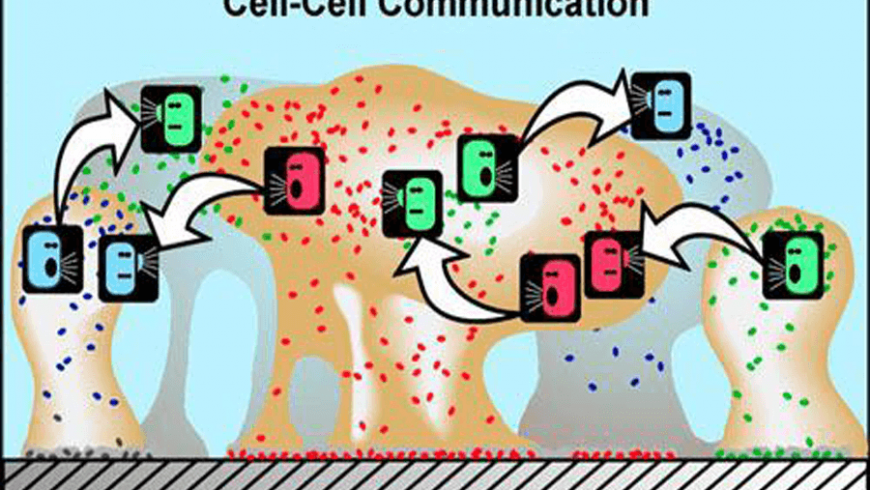
In the cartoon above, various species of bacteria are represented by different colors. Bacteria can produce chemical signals (“talk”) and other bacteria can respond to them (“listen”) in a process commonly known as cell-cell communication or cell-cell signalling. This communication can result in coordinated behaviour of microbial populations. (Source: Montana State University – Center for Biofilm Engineering –
https://www.cs.montana.edu/webworks/projects/stevesbook/contents/chapters/chapter001/section006/green/page003.html)
References
Bassler, B.L. and Losick, R. (2006). Bacterially speaking. Cell, 125: 237-246.
Bosgelmez-Tinaz, G. (2003). Quorum Sensing in Gram-Negative Bacteria. Turk J Biol., 27: 85-93.
Dubey, G.P. and Yehuda, S.B. (2011). Intercellular Nanotubes Mediate Bacterial Communication. Cell, 144 (4): 590. DOI: 10.1016/j.cell.2011.01.015
Dunny, G.M. and Leonard, B.A. (1997). Cell-cell communication in Gram-positive bacteria. Annu. Rev. Microbiol. 51: 527–564.
Engebrecht J.; Nealson K. and Silverman M. (1983). Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell, 32: 773–781
Freestone, P.P.E.; Sandrini, S.M.; Haigh, R.D. and Lyte, M. (2008). Microbial endocrinology: how stress influences susceptibility to infection. Trends in Microbiology, 16: 55-64
Fuqua, W.C.; Winans, S.C. and Greenberg, E.P. (1994). Quorum sensing in bacteria: the LuxR/LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176: 269–275.
Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S. and Ciofu, O. (2010). Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag., 35: 322–332
Lyte, M. (2004). Microbial endocrinology and infectious disease in the 21st century. Trends in Microbiology, 12: 14–20
Nealson, K.H. and Hastings, J.W. (1979). Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev. 43: 496–518
Reiche, E.M.V.; Nunes, S.O.V. and Morimoto, H.K. (2004). Stress, depression, the immune system and cancer. The Lancet Oncology, 5: 617–625
Roux, A.; Payne, S.M.; Gilmore, M.S. (2009). Microbial telesensing: probing the environment for friends, foes and food. Cell Host Microbe, 6: 115–124
Sansonetti, P.J. (2004). War and peace at mucosal surfaces. Nat. Rev. Immunol., 4: 953–964
Smith, R.S. and Iglewski, B.H. (2003). Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J. Clin. Investig., 112: 1460–1465
Yarwood, J.M.; Bartels, D.J.; Volper, E.M. and Greenberg, E.P. (2004). Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol., 186: 1838– 1850
Dr. Dilip Kumar Sarma Professor Department of Microbiology C.V.Sc., AAU, Khanapara, Guwahati