Introduction:
In India, about 20.5 million people depend on the livestock sector for their livelihood. It contributes around 4.11% GDP and 25.6% of total agricultural GDP of the country. Reproductive inefficiency is one of the major causes of economic loss in livestock industries. Assisted Reproductive Technology (ART) is a global term used to describe the infinite options of advanced infertility treatment. It refers to all technologies where gametes (sperm or ovum) are manipulated outside the body (in-vitro). Various ARTs have been developed and refined to obtain a large number of offspring from genetically superior animals or obtain offspring from infertile (or sub-fertile) animals in addition to disease control. ART includes Artificial Insemination (AI), In-vitro Embryo Production (IVP), Embryo Transfer (ET), Manipulation of Fertilization and multiplication techniques (cloning) for the application of transgenesis etc.
- Artificial insemination (AI):
AI, the first generation of reproductive biotechnology, comprises the collection, evaluation and preservation of semen and its manual or instrumental transfer into the female reproductive tract. AI is the least-invasive, low-cost, and most promising biotechnology for livestock production, companion animals, non-domestic animals, and endangered species and is a critically important for the maintenance of genetic diversity. In today’s agricultural industry in the developed countries, AI is used in about 80% of dairy cattle and more than 90% of breeding sows.
1.1 Freeze-Drying: Sperm freeze-drying allow easy and low-cost semen storage at supra-zero temperature (4-21 °C) without the need for liquid nitrogen. This method involves a multistep process including primary and secondary drying and two phase transitions to arrive at completely dried samples. It basically follows the principles of anhydrobiosis occurring in nature. To date, freeze-drying is deleterious to most sperm components including DNA, thus precluding its use for AI or IVF.
1.2 Cryopreservation of semen: Damage to cells during freezing and thawing strongly depends on the cooling rate: slow cooling (about 5 °C/min) results in dehydration due to hypertonic conditions induced by extracellular ice formation, whereas rapid cooling (>100 °C/min) leads to the formation of damaging intracellular ice crystals (Mazur, 1963). Typically, sperm diluted in freezing extenders are slowly cooled from room temperature to 5 °C at a rate of approximately −0.1 °C to −0.3 °C/min, followed by freezing at a rate of −10 to −60 °C/min down to a temperature of −80° or −120 °C, after which samples are plunged into liquid nitrogen (−196 °C).
1.3 Vitrification: It is the process of ultra-rapid freezing (2000°C/min) by direct exposure of extended semen/oocytes (gametes) samples with cryoprotectants to liquid nitrogen. Using this technique, samples instantly reach a glasslike state without formation of deleterious ice crystals.
1.4 Semen evaluation by Swim up: After freezing and thawing, about half of the sperm in a good semen sample are dead. After thawing, competent cells must be separated from dead or weak ones. Most straws are frozen with about 20 million motile sperm per dose so that they contain at least 10 million motile sperm per dose after thawing.
- Sex-Sorted Sperm in Domestic Farm Animals:
In mammals, sex is determined at fertilization by the sex chromosomes, X and Y. These are equally distributed among sperm, whereas the oocyte always carries an X chromosome. In the resulting zygote, the ‘XX chromosome’ combination determines a female and the ‘XY chromosome’ combination a male. The production of pre-sexed livestock by sperm or embryo sexing is a useful breeding tool to increase production efficiency, especially for traits that are sex related. In mammals, the most efficient method to bias sex ratios in offspring is to separate X and Y chromosome-bearing sperm by flow-cytometry before insemination. The quantitative methods, which differentiate between X and Y sperm on the basis of total DNA and then apply flow cytometric sorting, have been able to separate the two sperm populations with high accuracy. Sperm are labelled with a DNA fluorescent dye. After recognition and electric charging, droplets containing single sperm are deflected and pushed into a collection medium from which they are further processed. Sorted samples are collected in tubes pre-filled with collection medium. This set-up allows the identification and selection of individual sperm into populations with sort purities above 90% of the desired characteristics. The sex-sorting process can cause sperm damage. The main sources of damage are incubation with the fluorescent stain and exposure to the UV laser, mechanical forces and electrical charge.
2.1 Alternatives to Quantitative Flow Cytometry:
2.1.1 Qualitative Signal Creation Using Gold Nanoparticles (AuNPs): AuNPs have been assessed as suitable carriers for sequence-specific labeling of haploid mammalian sperm and for visualization and tracing with an annealed DNA probe.
2.1.2 Microfluidics: Based on the knowledge of flow cytometry, microfluidic sorting could provide a completely new approach to hydrodynamic cell orientation and charge-less deflection or impedance-related detection combined with a di-electrophoretic sorting.
2.1.3 Promotion of Naturally Occurring Genome Variations by Gene Editing: Genome editing has become a fast-growing research area since the development of sequence-specific nucleases and four different editing processes have been used: meganuclease, zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN) and clustered regularly interspaced short palindromic repeats (CRISPR).
- In-vitro Embryo Production:
In-vitro embryo production is carried out in four phases: a. In-vitro Maturation (IVM), b. Preparation of spermatozoa, c. In-vitro Fertilization (IVF), and d. In-vitro Development (IVD).
3.1 In-vitro Maturation (IVM): Oocyte collection can be done either surgically or manually from the ovaries of slaughtered animals. Follicles of diameter 3-5 mm are mostly used for oocyte collection. Oocytes with compact cumulus and corona are selected for culture. IVM of oocytes is conducted for 24 hrs at 38.5oC in presence of 5% CO2 in a CO2 incubator.
3.2 Preparation of Spermatozoa: Capacitated sperm is essential for in-vitro fertilization. After capacitation, acrosome reaction in the head of sperm allows the release of certain enzymes which helpin penetrating the zona pellucida of the oocyte.
3.3 In-vitro Fertilization (IVF): The collected mature oocytes are co-cultured with capacitated sperm and allowed to incubate at body temperature of animal for 6-24 hrs.
3.4 In-vitro embryo development: It is a component of IVF where in resultant embryos (Zygote) are allowed to grow for some time in an artificial medium. Culture is continued for 24 to 36 hrs before a further assessment for embryo cleavage is performed. At this stage, the embryos are normally between two to eight cell (blastomere) stages. Embryo quality is assessed depending on blastomere symmetry and the degree of cytoplasm fragmentation before transfer.
- Embryo Transfer Technology:
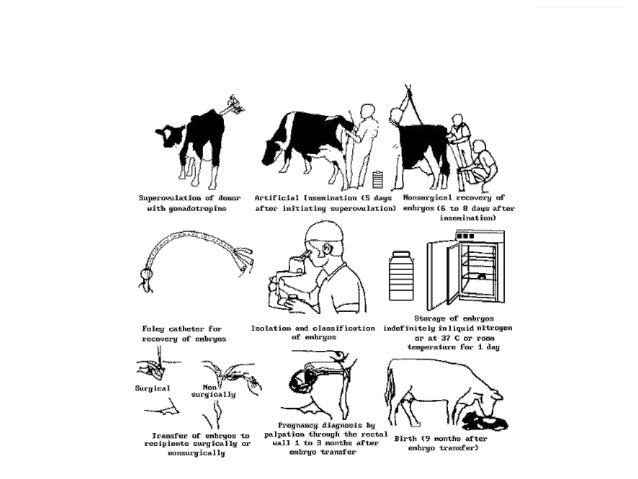
Embryo transfer is a specialized technique of breeding. A sexually mature female referred to as the donor is injected with exogenous hormones to produce more ova, which are fertilized inside her either by natural or artificial service. These are then removed prior to their implantation and transfered to the reproductive tract of synchronized surrogate mothers of the same species referred to as the recipients. ETT consists of the following important steps: Selection and management of donor and recipients, Synchronization of the estrous cycle of donor and the recipient animals, Superovulation of the donor and the recipients, Estrus should occur on fifth day of the process and all animals should be heat detected. Donor animals should be artificially inseminated 4-22 hrs after onset of estrus. It is followed by Collection of Embryos, Selection of embryos for transfer, and Transfer of embryos.
- Embryo Splitting:
Embryo splitting is the physical division of embryos to develop two or more genetically identical animals from a single embryo. Embryos develop through four distinct stages from fertilization through blastula. At the 4 to 8-cell stages, each cell of embryos has the totipotent capability to developing whole embryo. So it may be manually divided under the microscope to form two to four different groups of cells.
- Intra-cytoplasmic sperm injection (ICSI):
Injection of single mature immobilized normal spermatozoa into the cytoplasm of a mature metaphase II oocyte is known as intra-cystoplasmic sperm injection (ICSI). This technique is used to overcome severe male infertility. The procedure is performed by laparoscopy.
- Zygote Intra-Fallopian Transfer (ZIFT) & Gamete Intra-Fallopian Transfer (GIFT):
ZIFT, also referred to as Tubal Embryo Transfer (TET), is an ART technique in which embryos are transferred into the fallopian tubes for purposes of achieving pregnancy. Meanwhile, GIFT allows the transfer of gamete into the fallopian tubes. Both ZIFT and GIFT procedures require the female to have at least one functioning fallopian tube which is a disadvantage when compared with IVF.
- Cloning:
Cloning is a powerful technique and potentially it could be used for multiplication of elite animals and minimize the genetic variation in experimental animals. Cloning using somatic cells offers opportunities to select and multiply animals of specific merits. The first animal produced by somatic cell cloning (Somatic cell Nuclear Transfer) was a sheep called “Dolly”.
- Transgenesis:
Foreign DNA is introduced into early embryos in such a way that it becomes incorporated permanently into the host DNA. Several biotechnological techniques such as pro-nuclear micro-injection, cytoplasmic micro-injection, retrovirus based vectors, transferring DNA to embryos or embryonic stem cells via retroviral vectors, sperm mediated gene transfer of lenti-vectors and RNA interference are presently being used to produce transgenic animals.
Conclusion:
Assisted Reproductive Technology is the revolutionized way to overcome reproduction failure in farm animals. Different types of ART help to drastically accelerate the genetic improvement and uniformity of animal herds. The success of ART will no doubt continue to improve with higher pregnancy rates, reduced risks and complications related to reproduction and improve productivity. So, it is important that the international & national societies (SBTE, IETS) should support to the scientists and researchers in this field to develop and promote the different ARTs.
References:
- Heiner Niemann & Christine Wrenzycki (2018). Animal Biotechnology 1: Reproductive Biotechnologies. https://doi.org/10.1007/978-3-319-92327-7
- Hafez, E.S.E. (1993). Reproduction in Farm Animals, 6th ed. Philadelphia: Lea & Febiger.
Dr. Krishna Bharali & Dr. Joyshikh Sonowal
PhD Scholars, Department of Veterinary Biotechnology, ICAR-IVRI, Izatnagar, UP, India