Malaria has plagued humanity since antiquity. Today, it may sound surprising to recall that right until the late nineteenth century, malaria was believed to be a malady caused by foul odorous air or “miasmas” emanating from polluted water bodies, swamps or marsh lands. It was the French military surgeon and pathologist, Alphonse Laveran (1880), who discovered a protozoan parasite to be the cause of malaria; he called it Oscillariamalariae, which was later renamed as Plasmodium. Some years later in 1897, Ronald Rossincriminated mosquito as the vector responsible for transmission of malaria. (Both Laveran and Ross received Nobel Prize for their work). These studies marked the beginning of scientific pursuit and understanding of what turned out to be a very complex protozoan pathogen in terms of its fascinating basic biology and the terrible disease it inflicts on humanity. Ever since, scientists all over the world have been relentlessly working to unravel the molecular mechanisms involved in host-pathogen interaction as well as to devise effective strategies for treatment (drug development) and control (vector control) of malaria.
One of the most successful and impressive scientific advances in the 20th century in control of dreaded infectious diseases like small pox, polio, etc., has been the development of vaccines. No wonder that efforts towards developing a malaria vaccine have been in the forefront of R&D programmes of many leading labs across the globe. However, developing a malaria vaccine presents a host of formidable challenges. But before we get into the charm and challenge of developing a malaria vaccine, let us first revisit our basic knowledge about the life cycle of the malaria parasite. This knowledgewill help us to know the stages in the life cycle of the parasite which may be vulnerable to immune and/or drug attack, and hence attractive targets for interventional strategies.
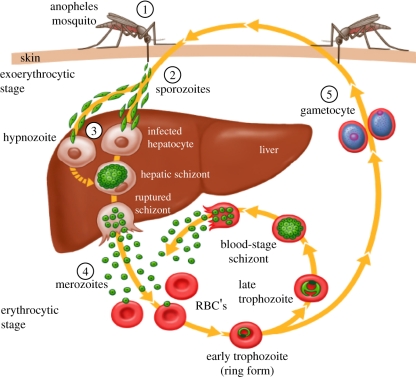
Typically, three distinct cycles constitute the complete life cycle of malaria parasite (Figure 1), namely, Exoeryhtrocytic Cycle in the liver of the vertebrate host, Erythrocytic Cycle in the red blood cells of the vertebrate host, and Sporogonic Cycle in the invertebrate host/vector (female Anopheles mosquito). Malaria infection in a vertebrate host starts with the bite by an infected female Anopheles mosquito, which in the process of taking its blood meal injects thousands of sporozoites, the infective form of malaria parasite, into the skin of the vertebrate host. Eventually sporozoites find their way into the blood stream and get carried away to the liver where they infect hepatocytes, and over a period ranging from days to years depending upon the species of the malaria parasite, each sporozoiteundergoes exponential division to give rise to what is called tissue schizont stage. Each tissue schizont eventually bursts and releases thousandsof motile merozoites into the blood stream, each ready to infect an erythrocyte and initiate asexual erythrocytic cycle of the parasite. This cycle comprises sequential differentiation of an intracellular merozoite into a signet ring stage and trophozoite which undergoes multiple cell divisions to give rise to a schizont containing up to 32 or even more merozoites. Eventually schizonts burst out to release merozoites, each ready to initiate fresh asexual erythrocytic cycle of the parasite. It is this asexual erythrocytic cycle of the parasite which is responsible for all the morbidity and mortality associated with malaria (Figure 1). After a certain number of erythrocytic cycles, some of the parasites decide to differentiate into sexual stages, i.e., male and female gametocytes to be taken up by the feeding female Anopheles mosquito. It is in the mosquito gut that parasite’s male and female gametes are formed, to accomplish the process of fertilization and followed by further development into sporozoites. The sporozoites move into salivary glands of the female mosquito, ready to infect fresh victim during the next blood meal of the mosquito (Figure 1).
As mentioned above, paroxysms, the fever episodes of malaria coincide with the bursting of infected erythrocytes and release of products of multiplication of the parasite in the blood stream. The periodicity and severity of fever is characteristic of different species of malaria parasite. Thus, four species of malaria parasite that can infect humans are as follows:
- Plasmodium falciparum, the most virulent species, cause of malignant tertian malaria (48 hr periodicity)
- vivax, the second most virulent species, causes benign tertian malaria (48 hr periodicity)
- malariae, causative agent of quartan malaria, (72 hr periodicity)
- ovale, largelysimilar to vivax
Figure 1. Life Cycle of malaria parasite (Source: CDC, Atlanta, Georgia, USA)
Developing a malaria vaccine involves surmounting multiple tough challenges (Table 1). The fact that more than one species of Plasmodium causes malaria in humans means that an effective vaccine has to be developed against each of those species; that represents the first challenge in the path to a successful malaria vaccine. Multiple stages of the life cycle add the next level of complexity to the problem. Multiple antigenic proteins present in each of the multiple stages of life cycle, genetic diversity and antigenic variation, redundancy in molecular mechanisms of host cell invasion, etc., amplify the challenge of developing a malaria vaccine by several folds.
|
Table 1. Multiplicity of Challenge in Developing a Malaria Vaccine
|
|
1. Multiple species of human malaria parasite 2. Multiple genetic strains of each species 3. Multiple stages of life cycle, each with its own specific niche 4. Multiple antigens in each stage of the parasite 5. Multiple variants of each antigen 6. Multiple “immunological decoys”: immunodominant but generally non-protective antigenic proteins or peptides 7. Lack of a reliable animal model system 8. Poor immunogenicity of potentially protective antigenic proteins 9. Shortlife-span of protective immune response 10. Poor immunological memory following malaria vaccination/infection
|
Efforts for developing malaria vaccine started way back in 1960s, when Ruth Nussenzweig and colleagues, demonstrated that immunization with attenuated sporozoites, delivered through bites of x-irradiated infected mosquitoes, imparted sterile protection to mice challenged with the murine malaria parasite P. berghei sporozoites (Nussenzweig et al., 1967, Nature, 216:160-61). It was soon followed by successful immunization of humans against P. falciparum (Clyde et al., 1973, Am J Med Sci, 266:169-177). These early studies, along with subsequent corroborating studies, in mice, non-human primates and humans established the benchmark of an effective malaria vaccine to be long term sterile immunity against Plasmodium challenge infection (Hoffman et al., 2002, J Infect Dis 185: 1155-64; Hoffman et al., 2015, Vaccine 33: D13-D23)
Ever since those pioneering promising studies, the research for malaria vaccine discovery/development has gone through cycles of euphoria and frustration, underlining scientific roadblocks like incomplete understanding of the biology of the parasite on the one hand, and nature of the “protective” immune response required on the other. As a result of application of advanced genomic biology and molecular immunology strategies over a period of more than last thirty years, a large number of molecules have been identified and evaluated as candidate vaccines. A number of pre-erythrocytic stages and asexual blood stages vaccine formulations are being evaluated in clinical trials as indicated in Table 2 and 3, and Figure 2.
Table 2. List of leading Pre-erythrocytic malaria vaccine candidates against P. falciparum malaria
| Sr. No. | Vaccine Types | Vaccine candidate/ Antigen (Developer/Promoter) | Molecular Characteristics | Best Protective Efficacy reported | Reference |
| 1. | Pre-Erythrocytic (PE) candidates | PfSPZ:
Radiation attenuated sporozoites: (Sanaria Inc., USA) |
Metabolically active, non-replicating Pfsporozoites | 48% in Phase 1/2a clinical trial
|
Lancet Infec Dis (2017)17:498-509 |
| Pf GAP3KO:
Genetically attenuated sporozoites with the three genes expressed in the pre-erythrocytic stage (Pf p52–/p36–/sap1–)deleted |
Metabolically active sporozoites, with their replication arrested in the liver | 100% efficacy in the proof of concept (POC) Study;
Phase 1 clinical trial underway |
Sci Transl Med. (2017 Jan 4), 9(371):eaad9099
|
||
| RTS,S/AS:
C-terminal part of PfCSP (RT)+Hepatitis B virus Surface Ag(S)/ Adjuvant System (AS) (GSK |
Recombinant chimeric protein co-expressed with Sprotein in the yeast, S.cerevisiae; forms Virus like particles (VLP) | Upto 36% in Phase 3 clinical trial;
Phase 4a clinical trial underway |
Lancet (2015) 386: 31-45 | ||
| Ad35.CS.01-RTS,S/AS01 | Human adenovirus 35 vectored CS and RTS,S/AS01, in a heterologous prime-boost strategy; priming with Ad35.CS and boosting with RTS,S/AS01
|
44% in the prime-boost group; 52% in the RTS,S/AS01 alone group in Phase 2 clinical trial;
No increase in efficacy over that with RTS,S/AS01 alone |
PLoS One.(2015)
10:e0131571. |
||
| ChAd63/MVA ME-TRAP:
Chimpanzee Adenovirus 63/ Modified Vaccinia Ankara (MVA) expressing a string of Multiple epitopes (ME)fused with thrombospondin-related adhesion protein (ME-TRAP) of P. falciparum |
Priming with ChAd63 encoding ME-TRAP, boosting with MVA encoding ME-TRAP; ME comprises a string of 17 B cell and T cell epitopes from six different P. falciparum preerythrocytic antigens plus a single CD8+ T cell epitope of P. berghei | Safety and Immunogenicity studies in Phase1/2 clinical trials
|
Mol Ther. (2016) 24: 1470–1477. | ||
| No vaccine efficacy was observed | PLoS One.(2016)
11:e0167951. |
||||
| 67% reduction in the risk of infection | Sci Transl Med (06 May 2015) 7: 286re5 |
Table 3. Selected Blood-stage vaccine formulations in clinical trials (Phase 1/2)
| S. No. | Antigen Formulation | Developer | Clinical Trial Phase | Reference |
| 1. | EBA175 RII/aluminium phosphate | NIAID, NIH | 1 | https://clinicaltrials.gov/ct2/
show/NCT00347555 |
| 2. | GMZ2 field/Alhydrogel© | AMANET, Serum Statens Institute | 2 | Vaccine. (2010) 28: 6698–6703. |
| 3. | PfAMA1-DiCo/GLA-SE or Alhydrogel® | INSERM | 1 | Vaccine. (2017) 35: 6218-6227. |
| 4. | MSP3-LSP/AlOH | European Vaccine Initiative, AMANET | 2 | Vaccine. (2007) 25: 2723-2732.
https://core.ac.uk/download/pdf/ 151539143.pdf
Vaccine. (2016) 34: 2915-2920 |
| 5. | ChAd63 MSP1/MVA MSP1 | University of Oxford | 1 | Molecular Therapy (2011) 19: 2269–2276 |
| 6. | ChAd63 RH5 ± MVA RH5 | University of Oxford | 1 | JCI Insight. (2017) 2: e96381. |
| 7. | ChAd63 AMA1/MVA AMA1 | University of Oxford | 1 | PLoS ONE (2012) 7: e31208. |
EBA, erythrocyte binding antigen; GMZ2, a recombinant fusion protein of conserved regions of glutamate rich protein (GLURP) and merozoite surface protein (MSP)-3; PfAMA1-DiCo, Plasmodium falciparum (Pf) Apical Membrane Antigen 1 Diversity Covering vaccine; RH5, Pf reticulocyte–binding protein homolog 5; MVA, modified vaccinia virus Ankara; ChAd63, Chimpanzee adenovirus 63.
Figure 2. Global Malaria Vaccine Pipeline
However, it is evident that currently, out of a large number of malaria vaccine candidates in pipeline (Figure 2), only one vaccine, namely the pre-erythrocytic vaccine RTS,S or Mosquirix”, has completed the mandated clinical trials, and has been approved for human use. The RTS is a chimeric protein, comprising of carboxy-terminal half of P. falciparum circumsporozoite protein (CSP) containing known repetitive B cell epitopes and T cell epitopes (termed as RT), genetically fused to hepatitis B virus surface antigen (Figure 3). As mentioned above, Nussenzweig and colleagues had earlier established CSP as a potential vaccine candidate.
Figure 3. Schematic of circumsporozoite protein and the RTS,S construct (from Vaccine 33 (2015) 7425–7432)
The RTS,S is made up of two proteins, namely the chimeric protein RTS, and the hepatitis B virus surface antigen S, expressedsimultaneously in the genetically engineered Saccharomyces cerevisiae yeast cells; this strategy makes use of the earlier observation that large abundance of S component in the vaccine allows it to form putatively immunogenic virus like particles (VLPs). Nevertheless, it may be pertinent to point out that RTS,S alone was found to be poorly immunogenic. But when given in combination with an advanced adjuvant system AS-01 or AS-02, RTS,S could provide significant protection against experimental malaria in mice and non-human primates. It is interesting that although this vaccine was developed for prevention of infection (by neutralizing sporozoite stage of the parasite), it was also found to mitigate severity of disease as an important collateral benefit.
Thus, we can see that although malaria vaccine discovery research and development is the focus of intensive investigation by several leading research labs in the world, only a handful of candidates have reached clinical trial stage. Malaria infection is known to induce rather poor immunity in humans, in spite of repeated and prolonged malaria infections over several years. This naturally acquired partial immunity fails to provide sterile protection and is rather short-lived; furthermore, this immunity is strain- and stage-specific in its effect. So obviously, for a potent and really effective malaria vaccine, one has to come up formulations and scientific strategies which improve upon nature. And that remains a daunting task as of today.
Dr. Pawan Sharma
DBT’s Visiting Research Scientist
Department of Animal Biotechnology
C.V.S.c., A.A.U.,Khanapara, Guwahati-22