Recent advances in the gut microbial research have shed the light on the importance of gut microbiota and its contribution in maintaining our health. The gut microbiota maintain a homeostasis in the body and thus contribute to metabolism and immunity development, and any perturbation in the gut microbial homeostasis leads to diseases. Involvement of gut microbiota in the disease development is not only restricted to localised effects as in inflammatory bowel disease but it can also effect at distant sites such as liver, heart, brain and the hematopoietic system.
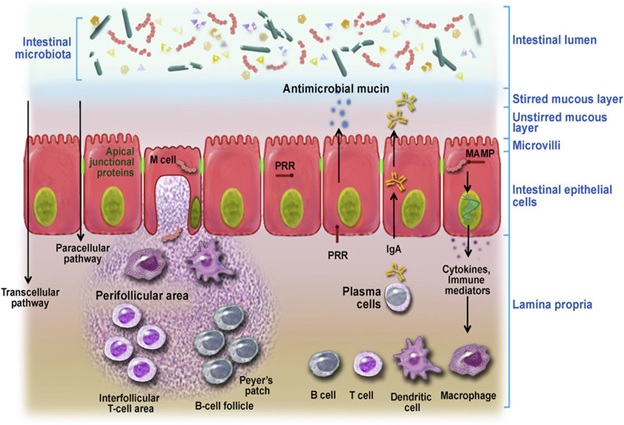
The intestinal barrier is an integral part of the gastrointestinal tract which comprises several physical and immunological layers. Specifically, the intestinal barrier (Figure 1) comprises of i) Mucus layer that contains commensal microbiota, secretory immunoglobulin A (IgA) and anti-microbial peptides, ii) The epithelial intestinal layer which acts as a physical barrier that contains tight junctional complexes, adherens junctions and desmosomes between intestinal epithelial cells (IECs), and finally iii) Lamina propria with its resident population of innate and adaptive immune cells such as T and B cells, macrophages, dendritic cells of Peyer’s patches and mesenteric lymph nodes that make up the intestinal immune barrier1. Physical intestinal barrier prohibits bacterial translocation from the lumen and allows selective absorption of critical nutrients required by the host, and any perturbation to this tightly regulated intestinal barrier could lead to a leaky gut and allow bacterial endotoxins to penetrate the mucosa and enter the systemic circulation. Alcohol consumption is reported to disrupt the functional and structural integrity of IECs contributing to increased gut leakiness by a variety of mechanisms2,3. After consumption, alcohol is absorbed by different cells in the mouth, stomach and intestine and only a small amount is metabolized in the cells. However, a majority of alcohol that is consumed passes directly into the blood stream due to its hydrophobicity. Alcohol travels through blood to the liver where it undergoes metabolism. A number of enzymes are involved in alcohol metabolism, the most well-known are Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the liver4. Initially, ADH converts alcohol into acetaldehyde which is a highly toxic carcinogen. Acetaldehyde promotes alcohol dependence and is found in all the regions of the brain following alcohol consumption. Acetaldehyde is highly pro-inflammatory; it activates complement cascade recruiting neutrophils and eliciting production of reactive oxygen species (ROS)5. Though highly toxic, acetaldehyde is quickly metabolized by ALDH to form acetate, which is then finally converted to carbon dioxide and water that can be removed from the body easily.
Alcohol and the intestinal microbiota
Alcohol is known to alter the intestinal microbiome by altering the intestinal microenvironment that favours the growth of pathogenic bacteria over that of non-pathogenic commensal bacteria. An alteration in function and composition of colonic microbiome was reported in alcoholic patients with reduction in Bacteroidetes and increase in Proteobacteria6. Chen et al.7 also reported an increase in Proteobacteria along with increased prevalence of Fusobacteria in hepatitis B virus (HBV) and alcohol related cirrhotic patients. Alcohol mediated over-growth of intestinal bacteria and dysbiosis was reported in conventional mice with an increased prevalence of Enterobacteriaceae8. Studies on mice reported relative abundance of Bacteroidetes and Verrucomicrobia in alcohol-fed mice compared to control mice with relative predominance of Firmicutes9. Over-growth of Akkermansia muciniphila, a mucin degrader, was also reported in alcohol-fed mice10.
Increase in ROS following ethanol exposure contributes to disruption in intestinal homeostasis and hypothalamic homeostasis by increasing the oxidative stress through nitric oxide (NO). At the normal level, NO is involved in maintaining normal intestinal barrier function11. However, when NO is in excess after chronic alcohol consumption, it results into barrier disruption and increased gut leakiness12. Thus destruction of the barrier integrity of the intestinal epithelium caused by alcohol abuse could allow translocation both bacteria and their products including lipopolysaccharides (LPS) from the gut to the circulation13 resulting in systematic inflammation. Alcohol also has a stimulatory effect on neuroendocrine hormones such as Cortico-trophin releasing hormone (CRH), which can lead to an increase in gut permeability14. Circulating LPS was reported to increase the pro-inflammatory cytokines, TNF-α in the brain, liver and serum15 while anti-inflammatory cytokines IL-10 was reported to decrease in both brain and intestinal tissues16. Thus alcohol consumption causes a direct effect on the brain and the intestine by inducing neuro-inflammation and intestinal inflammation, respectively. Interestingly, germ-free mice showed reduced liver pathology after alcohol administration compared to that in conventional mice which showed the involvement of gut microbiota in the development of alcoholic liver diseases.
Alcohol and the gut brain axis
Sudo et al.17 reported for the first time the link between the intestinal microbiota and the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is extremely important in generating a proper stress response and is slowly developed through adolescence. Germ-free mice do not have properly develop HPA axis. Alcohol has been shown to activate the HPA axis leading to an increase in corticosteron in the circulatione in mice and rats following a single binge18. Many research groups have shown profound effects of heavy alcohol drinking from young age on the brain and development of depressive like behaviour19,20, while altered bacterial populations in the gut appears to have numerous negative effects on the CNS21,22. On the contrary, probiotics treatment can reduce the psychological stress induced due to alcoholism23. Intestinal microbes can directly interact with the CNS through vagus nerve stimulation and enteric nervous system24. Studies have reported that the exposure of certain gram negative bacteria that induce inflammation can lead to changes in the neuronal activity in the hypothalamus and central brain regions25. Other than bacteria, bacterial metabolites can also influence inflammation and neuronal signalling in the CNS26. The gut bacteria has the ability to alter the neurochemical signals such as brain-derived neurotrophic factors (BDNF), gamma-aminobutyric acid (GABA) and serotonin. A reduced expression of BDNF proteins was reported in mice with anxiety like behaviour27. Deregulation of GABA signalling was reported during anxiety and depression like behaviour28. Gut is one of the largest producers of serotonin in the body and intestinal dysbiosis can lead to altered serotonin expression which can effect neuronal signalling of the brain29.
Alcohol and Gut-liver axis
The gut liver axis is a major pathway for development and progression of alcoholic liver diseases. Liver plays an essential role in modulation of gut microbiota and its effect through multiple functions, including bile acid production and enterohepatic circulation as well as exposure to gut bacterial end-products along with nutrients via the portal vein30. Liver does not only receive nutrient-rich blood from the intestine, but also acts as the first target for the intestinal microbiota through microbe-associated molecular patterns (MAMPs) which can elicit inflammatory responses via pattern recognition receptors (PRPs) and microbial metabolites. The multilayer intestinal barrier protects the liver from exposure to pro-inflammatory MAMPs. However, alteration in the gut bacteria and failing gut barrier in chronic liver diseases (CLD) contribute to chronic inflammation and progression of liver diseases31,32 and thereby increase the risk of hepatocellular carcinoma (HCC). Liver produces bile acids which is released into the duodenum in conjugated form, where they undergo microbial modification and participate in the enterohepatic cycling through the farnesoid X receptor (FXR)-fibroblast growth factor 19 (FGF-19) axis that can modulate the gut barrier and an integral part of the gut-liver axis.
Figure 1: Intestinal barrier as modulator of intestinal homeostasis. Intestinal barrier is equipped with both physical and immunological barrier system. (Figure is adapted from Vanner et al., 2016)
Download PDF
References:
- Vancamelbeke, M. and Vermeire, S., 2017. Expert review of gastroenterology & hepatology, 11(9), pp.821-834.
- Banan, A et al., 1999. Journal of Pharmacology and Experimental Therapeutics, 291(3), pp.1075-1085.
- Tang, Y., et al., 2008. Alcoholism: Clinical and Experimental Research, 32(2), pp.355-364.
- Cederbaum, A.I., 2012. Clinics in liver disease, 16(4), pp.667-685.
- Setshedi, M., et al., 2010. Oxidative medicine and cellular longevity, 3(3), pp.178-185.
- Mutlu, E.A., et al., 2012. American Journal of Physiology-Gastrointestinal and Liver Physiology, 302(9), pp.G966-G978.
- Chen, Y., et al., 2011. Hepatology, 54(2), pp.562-572.
- Canesso, M.C.C., et al., 2014. BMC microbiology, 14(1), p.240.
- Yan, A.W., et al., 2011. Hepatology, 53(1), pp.96-105.
- Derrien, M., et al., 2008. Environ. Microbiol.74(5), pp.1646-1648.
- Alican, I. and Kubes, P., 1996. American Journal of Physiology-Gastrointestinal and Liver Physiology, 270(2), pp.G225-G237.
- Banan, A., et al., 2000. Journal of Pharmacology and Experimental Therapeutics, 294(3), pp.997-1008.
- Bode, C. and Bode, J.C., 2003. Best practice & research Clinical gastroenterology, 17(4), pp.575-592.
- Soderholm, J.D. and Perdue, M.H., 2001. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280(1), pp.G7-G13.
- Simi, A., et al., 2007. Interleukin-1 and inflammatory neurodegeneration.
- Qin, L., et al., 2008. Journal of neuroinflammation, 5(1), p.10.
- Sudo, N., et al., 2004. The Journal of physiology, 558(1), pp.263-275.
- Li, X., et al., 2006. Journal of leukocyte biology, 80(2), pp.367-375.
- Allen, C.D., et al., 2011. Neuroscience, 182, pp.162-168.
- Evans, B.E., et al., 2012. Addiction, 107(2), pp.312-322.
- Benton, D., et al., 2007. European journal of clinical nutrition, 61(3), p.355.
- Rao, A.V., et al., 2009. Gut pathogens, 1(1), p.6.
- Messaoudi, M., et al., 2011. British Journal of Nutrition, 105(5), pp.755-764.
- Forsythe, P., et al., 2014. Springer, New York, NY.
- Goehler, L.E., et al., 2008. Brain, behavior, and immunity, 22(3), pp.354-366.
- Stilling, R.M., et al., 2014. Genes, Brain and Behavior, 13(1), pp.69-86.
- Bercik, P., et al., 2011. Gastroenterology, 141(2), pp.599-609.
- Cryan, J.F. and Kaupmann, K., 2005. Trends in pharmacological sciences, 26(1), pp.36-43.
- Heijtz, R.D., et al., 2011. Proceedings of the National Academy of Sciences, 108(7), pp.3047-3052.
- Bajaj, J.S., et al., 2019. Hepatology.
- Rooks, M.G. and Garrett, W.S., 2016.. Nature Reviews Immunology, 16(6), p.341.
- Tremaroli, V. and Bäckhed, F., 2012. Nature, 489(7415), p.242.
- Vanner, S.J., et al., 2016. Gastroenterology, 150(6), pp.1280-1291
Dr. Madhusmita Dehingia
DBT-RA, Animal Biotechnology Laboratory
College of Veterinary Science, Khanapara