Author Information:
Name of the Author/Authors: Dr. Barnali Deka
Designation of Author/Authors: Guest teacher, Department of Chemistry, Handique Girls’ College.
E-mail ID of Author/Authors: barnalideka.bd@gmail.com, Ph. No. 9957586575.
Name and address of the Institute to which the Author/Authors are associated: Handique Girls’ College, GNB Road, Dighalipukhuri, Guwahati-781001.
Halide ions occur abundantly in Nature and are often associated with vital chemical and biochemical processes (Suzukia et al., 2006; Stolting et al., 2014). Among the halides, bioavailability of Fˉ anion (1.3 ppm) is lower in sea water relative to Clˉ anion (20,000 ppm) and Brˉ anion (70 ppm). Even the least abundant halide ion, i.e. Iˉ anion (0.02 ppm in surface water), had been found in ~ 120 iodine-containing natural products as it is easily oxidized by haloperoxidases to iodonium ion (I+) (Kazuaki, 1997; Pee and Curr, 2012; Vaillancourt et al., 2006).
Fˉ anion is the 13th most abundant element in Earth’s crust, mostly found in ground water. Fˉ anion has the highest enthalpy (~120 kcal/mole), which creates problem in nucleophilic catalysis from water, as the enzyme has to evolve a desolvation strategy (Furuya et al., 2011). The haloperoxidases are unable to oxidize Fˉ anion because the oxidation potential of hydrogen peroxide (-1.8 eV) is lower than that of Fˉ (-2.87 eV) but higher than that of the other halogens (Clˉ = -1.36 eV; Brˉ = -1.07 eV; Iˉ = -0.54 eV) (Ballschmiter, 2003). These anomalous physical properties of Fˉ anion have limited the evolution of fluorine biochemistry and only 21 biosynthesized fluorine containing natural molecules are known, compared to thousands of chlorine and bromine containing homologues.
Large diversity of halogenated compounds has been found in the environment. Different halogenating enzymes like haloperoxidases, halogenases, etc. play a major role in biocatalytic incorporation of halogen into natural products and biomolecules (Perrin, 2007). Haloperoxidases such as chloroperoxidases catalyse the halogenation reaction, where they may directly involve halide anions (Clˉ, Brˉ or Iˉ but not Fˉ) or chlorite (ClO2ˉ) or hypohalous acid (HOX) as halogenating agents (Van pee, 2003; Hofrichter and Ullrich, 2006; Muffler et al. 2007). They differ by the cations present in the prosthetic group which mostly contain heme iron or vanadate co-factor (Vaillancourt et al., 2006).
Interestingly, halogenases like α-KG-dependent halogenases and flavin dependent halogenases are capable of catalysing the regioselective carbon-halogen bond formation (Hasegawa et al., 1999; Keller et al., 2000; Hagan et al., 2002).
The fluorinating enzyme was first isolated from the soil bacterium Streptomyces cattelya and subsequently three more fluorinating enzymes had been identified (Sanada et al., 1986; Deng et al., 2002; Huang et al., 2014). When the bacterium grows in a medium containing Fˉ anion, the secondary metabolism produce bioactive fluorinated natural products (Schaffrath et al., 2003). For instance, it was showed that fluorinase catalyzed the nucleophilic substitution reaction of 5՛-carbon of the ribose ring of S-adenosyl-L-methionine (SAM) involving SN2 pathway (Scheme 1) to generate 5′-fluorodeoxyadenosine (5′-FDA) and L-methionine (Flury and Papritz, 1993; Schaffrath et al., 2003; Deng et al., 2014).
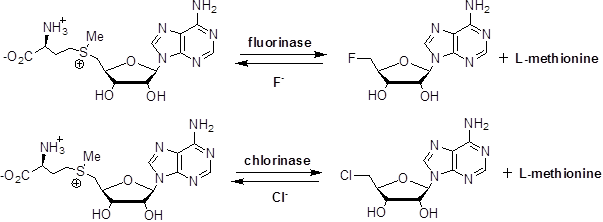
Scheme 1: Reactions of the fluorinase and the chlorinase, catalysing nucleophilic attack to C-5of SAM but with different nucleophiles (Deng et al., 2014).
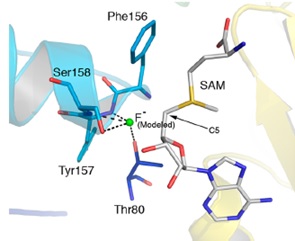
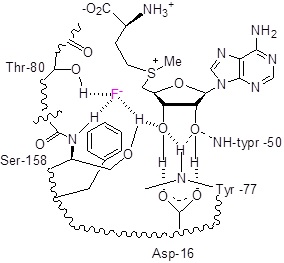
Figure 1: Structure of the active site of fluorinase showing the binding of Fˉ and SAM prior to the displacement reaction: (a) crystal structure, (b) model structure.
Clˉ ion is essential to human health and is transported across cell membranes by various proteins. It maintains osmotic pressure and pH across cell membrane and helps in restoration of normal acid-base and potassium equilibrium. Numerous applications in industry ranging from anti-freezing agents to metal processing require the use of halide ions. Although occur naturally, Fˉ and Brˉ anions may be environmental pollutants, both of them being toxic at high concentration (Chiun, 1996; Vengosh and Pankratov, 1998; Ayoob and Gupta, 2006; Peckham and Awofeso, 2014). Brˉ anion is an undesired by-side product of a number of industrial chemical processes (Aumont and Tressol, 1986; Haldimann et al., 1998). It is used as a sedative and anticonvulsant in medicine and in photographic industry. Iˉ anion is involved in thyroid physiology, photo electrochemical solar cells and as a cofactor in many antimicrobial actions (Jalali et al, 2005).
Previous studies suggested that low levels of Fˉ anion can benefit dental health and can widely be used for the prevention of dental caries, in treatment of osteoporosis and water purification (Kleerekoper, 1998; Loe, 2000). It is present in many products associated with oral hygiene and in a number of minerals (Kissa, 1997; Geddes, 2001). However, excessive ingestion may result in fluorosis, nephrotoxic changes, urolithiasis and even kidney disorders (Harper and Hagan, 1994; Cittanova, 1996; Siener and Hesse, 2003; Deng et al., 2004).
Conclusion:
The halide anions are very much essential in our day to day life from environmental, biological and chemical points of view. They play a significant role in several biochemical reactions. Thereby it’s important to study these anions and their utility.
References:
Aumont, G. and Tressol, J.C. (1986). Analyst 3: 841.
Ayoob, S. and Gupta, A.K. (2006). Crit. Rev. Environ. Sci. Technol. 36(6): 433-487.
Ballschmiter, K. (2003). Chemosphere 52: 313–324.
Chiun, L.C. (1996). Handbook of Chemical and Biological Sensors, Taylor & Francis.
Cittanova, M.L.; Lelongt, B. and Verpont, M.C. (1996). Anesthesiology, 84: 428–435
Deng, H.; Ma, L.; Bandaranayaka, N.; Qin, Z.; Mann, G.; Kyeremeh, K.; Yu, Y.; Shepherd, T.; Naismith, J.H. and O’Hagan, D. (2014). Chem. Biochem. 15: 364-368.
Deng, H.; O’Hagan, D. and Schaffrath, C. (2004). Nat. Prod. Rep. 21: 773.
Flury, M. and Papritz, A. (1993). J. Environ. Qual. 22: 747-758.
Furuya, T.; Kamlet, A.S. and Ritter, T (2011). Nature. 473: 470-477.
Geddes, C.D. (2001). Meas. Sci. Technol. 12: R53-R88
Haldimann, M.; Zimmerli, B.; Als, C. and Gerber, H. (1998). Clin. Chem. 44: 817
Harper, D.B. and O’Hagan, D. (1994). Nat. Prod. Rep. 11: 123
Hasegawa, M.; Yamada, K.; Nagahama, Y. and Somei M.B. (1999). Heterocycles 51: 2815−2821.
Hofrichter, M. and Ullrich, R. (2006). Appl. Microbiol. Biotechnol. 71: 276−288.
Huang, S.; Ma, L.; Tong, M. H.; Yu, Y.; O’Hagan, D. and Deng, H. (2014). Org. Biomol. Chem. 12: 4828.
Jalali, F.; Rajabi, M.J.; Bahrami, G. and Shamsipur, M. (2005). Anal. Sci. 21: 1533.
Kazuaki, I. (1997). Anal. Chem. 69: 3628.
Keller, S.; Wage, T.; Hohaus, K.; Hölzer, M.; Eichhorn, E. and van Pee K.H. (2000). Angew Chem. Int. Ed. Engl. 39: 2300−2302.
Kissa, E. (1987). Clin. Chem. 33: 253-255.
Kleerekoper, M. (1998). Endocrinol. Metab. Clin. North. Am. 27: 441-452.
Loe, H. (2000). International Dental Journal. 50: 129-139
Muffler, K.; Retzlaff, S.; van Pee, K.H. and Ulber, R. (2007). J. Biotechnol. 127: 425−433.
O’Hagan, D.; Schaffrath, C; Cobb, S.L; Hamilton, J.T.G. and Murphy, C.D. (2002). Nature, 416: 279.
Peckham, S. and Awofeso, N. (2014). Sci. World J. 1.
Pee, V. and Curr, K.H. (2012). Org. Chem. 16: 2583
Perrin, C.L.; Rodgers, B.L. and O’Connor, J.M. (2007). J. Am. Chem. Soc. 129: 4795-4799.
Sanada, M.; Miyano, T.; Iwadare, S.; Williamson, J.M.; Arison, B.H.J.; Smith, L.; Douglas, A.W.; Liesch, J.M. and Inamine, E. (1986). J. Antibiot. 39: 259.
Schaffrath, C.H.; Deng, D. and O’Hagan. (2003). FEBS Lett. 547: 111.
Siener, R and Hesse, A. (2003). European Journal of Clinical Nutrition, 57(Suppl 2): S47–S51.
Stolting, G.; Fischer, M. and Fahlke, C. (1997). Frontiers in Physiology. 5: 1-18.
Suzukia, M.; T. Moritaa and T. Iwamotob (2006). Cellular and Molecular Life Sciences, 63(1): 12–24
Vaillancourt, F.H.; Yeh, E.; Vosburg, D.A.; Tsodikova, S.G. and Walsh, C.T. (2006). Chem. Rev. 106: 3364−3378.
Vaillancourt, F.H.; Yeh, E; Vosburg, D.A.; Tsodikova, G. and Walsh, C.T. (2006). Chem. Rev. 106: 3364.
Van Pee, K.H. and Unversucht, S. (2003). Chemosphere. 52: 299−312.
Vengosh, A. and Pankratov, I. (1998). Ground water. 36(5): 815-824.