Dr. Shantanu Tamuly
Assistant Professor
Department of Veterinary Biochemistry
College of Veterinary Science, Assam Agricultural University
Khanapara, Guwahati-781022
The advent of non-live vaccines such as whole organism killed vaccines and subunit vaccines have opened a new avenue for the need of efficient adjuvant systems as the former is incapable of eliciting sufficient protective and long lasting immune response (1). As per the description of Cox and Coulter (2), there are five modes of action of adjuvants namely immuno-modulation, presentation, cytotoxic T-lymphocytes induction, antigen targeting and depot generation.
The immuno-modulation includes the regulation of cytokine network for better stimulation of immune system. The antigen presentation indicates the presentation of antigen to immune cells such as antigen presenting cells in the native immunogenic conformation. Activation of cytotoxic T-lymphocytes is required for intracellular pathogens such as Salmonella, Mycobacterium, protozoal pathogens, viruses etc. The antigen targeting indicates the capability of adjuvants to deliver to antigen presenting cells. The depot generation refers to storage of antigens near the site of injection, and slow and continuous release of antigen into the systemic circulation leading to long term maintenance of immune response. Different adjuvants follow one or more of the above mentioned mechanisms of action. In the recent years, the nanoparticle based vaccine adjuvant systems have gained centrestage (3). It is assumed that most of the nanoparticle based vaccine adjuvant systems utilizes the mechanism of efficient depot formation and improved antigen targeting (4).
Methods of preparation of nanoparticles:
The method of preparation of nanoparticles varies with the chemistry of compounds. However, they can be broadly classified as bottom-up and top-down methods (5). The top-down method involves breakdown of bigger particles into smaller size particles such as exposure of high energy radiation (e.g. ultrasonic waves) (Fig. 1). On the other hand, the bottom-up method involves the aggregation of molecules from the individual molecular stage up to the stage when the particle size reaches up to 50 to 100 nm (Fig. 2).
The methods of synthesis of nanoparticles can be broadly classified into three categories, viz. physical, chemical and biological methods. The physical method includes Arc discharge method, electron beam lithography, ion implantation, vapour phase synthesis and spray pyrolysis. The chemical method includes co-precipitation method, chemical reduction of metal salts, micro-emulsion method, pyrolysis, phytochemical method, sono-chemical method, sol-gel method, solvo-thermal method and phytochemical method. The biological methods include the use of micro-organisms, plant extracts, or the industrial wastes for synthesis of nanoparticles (6). The factors that influence the nanoparticle formation includes pH, temperature, type of buffer used, concentration of molecules, and time and speed of stirring (6,7). The nanoparticle synthesis requires standardization in each laboratory set up using design of experiments (DOE) such as full factorial design, Taguchi design of experiments or response surface methodology (RSM) that involves different levels of above mentioned factors. The DOEs based on Taguchi’s method (8–11) and RSM (12–15) are commonly used for process optimization as these are less time-consuming and economical. The bottom up method appears to be more convenient for biological purposes as these are biomolecule friendly. On the other hand, the use of ultrasonic waves may lead to degradation of loaded biomolecules on the nanoparticles. The nanoparticles are quite unstable given to their higher surface to volume ratio and consequently possessing higher surface energy. As per the second law of thermodynamics, the molecules that possess higher energy are unstable (16) because of which the particles tend to aggregate leading to formation of bigger particles. The nanoparticle aggregation can be prevented by incorporation of surfactants that stabilizes the nanoparticle and prevent their aggregation (17–19) such as c-TAB, citric acid, poly-venyl alcohol etc.
Classification of nanoparticle based adjuvants:
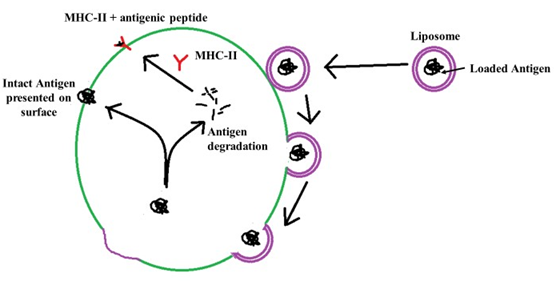
The nanoparticle adjuvants are classified as inorganic nanoparticles, polymeric nanoparticles, liposomes and virus like particles (4). The inorganic nanoparticles commonly used are calcium phosphate nanoparticles (20–22), aluminium hydroxide nanoparticles (23), silica nanoparticles (24), gold nanoparticles (25), etc. The inorganic nanoparticles are comparatively easy to prepare. They protect the vaccine antigen from degradation and efficient delivery to antigen presenting cells. The important hitch in the use of inorganic nanoparticles includes the possibility of toxicity. The calcium phosphate nanoparticles are reported to interfere with cell cycle of cultured human ovarian granulosa cells and consequently induced apoptosis (26). However, no report of infertility induced by calcium phosphate nanoparticle could be found by the author. There is a report of induction of immunotoxicity by the silica nanoparticles (27). The polymeric nanoparticles are basically the carbohydrate based nanoparticles that have wide applications in vaccine and drug delivery system for last three decades. Among the various polymers, the chitosan (28) and poly-lactide co-glycolide (PLG) (29,30) are most commonly used. These polymers are biodegradable and do not pose any deleterious residual effect in the host. The poly-lactide co-glycolide degrades into lactic acid and glycolic acid, both of which are normal constituent of the body. The chitosan is mainly degraded by lysozymes to non-toxic oligosaccharides that can be utilized by the host or can be excreted from the body (31). Another advantage of poly-lactide co-glycolide microparticle and chitosan nanoparticle is that their functional groups can be modified to manipulate their behaviour inside the host and their capability to carry the vaccine antigen (4). The liposome based nanoparticles are second widely used vaccine delivery systems. These liposomes assume bilayered vesicles upon hydration forming an aqueous core (Fig. 3). The antigens can be loaded into the aqueous core of the liposomes. The liposomes get merged with the cell membrane antigen presenting cells of the host releasing the entrapped antigen into the cytoplasm that further follows the path of MHC and intact antigen display for stimulation of cell mediated immunity and humoral immunity, respectively (Fig. 4). The virus like particles (VLPs) are non-infectious viral membranes that does not contain the genetic materials. These VLPs can be fused with multiple antigens or purified epitopes of vaccines for use as multi-epitope vaccine that can be used to immunize against many infectious agents (32).
Conjugation of nanoparticles with vaccine antigen:
The conjugation of vaccine antigens to nanoparticles is influenced by various factors such as hydrophobicity, surface charge of nanoparticle and antigen, pH of the conjugation medium, temperature, speed of stirring and ratio of conjugating nanoparticle and antigens. The optimum conditions for proper conjugation require prior knowledge of nature of antigens and the nanoparticles. For instance, the conjugation efficiency would be poor if the antigen is hydrophobic (such as bacterial outer membrane proteins) and nanoparticles are hydrophylic (such as calcium phosphate nanoparticles). Similarly, the conjugation of the purified proteins are used as a vaccine antigens having generally negative charge and the nanoparticles having negative surface charge would lower the conjugation efficiency. The optimum conditions for conjugation can be determined by the DOEs mentioned above. Some of the processes require the antigen incorporation during the formation of nanoparticles (33) or may be incorporated later. In the later approach, antigens are loaded into the nanoparticles simply by physically reacting them. This approach appears to be biomolecule friendly as in this approach the antigen is not exposed to extreme conditions.
Conclusion:
The use of nanoparticles as a vaccine adjuvant has brought a new revolution in the field of adjuvant technology. They have a vital role to play as vaccine and drug delivery systems. However, the concerns related to toxicity due to nanoparticles should be addressed.
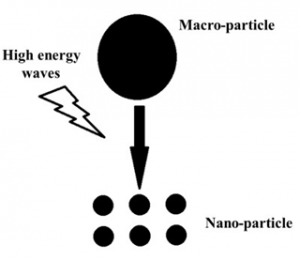
Fig 1. Synthesis of nanoparticles using top-down method
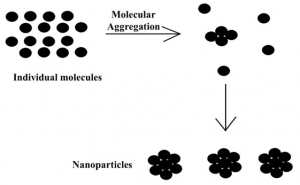
Fig 2. Synthesis of nanoparticles using bottom-up method
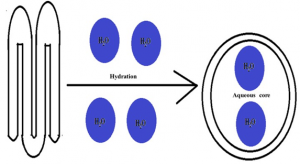
Fig 3. Formation of liposomes by hydration
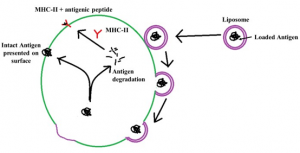
Fig 4. Delivery antigen loaded in the liposomes to the antigen presenting cell and subsequent presentation of antigen through MHC class II and MHC independent pathway
REFERENCES:
- 1. Awate S, Babiuk LA, Mutwiri G. Mechanisms of Action of Adjuvants. Front Immunol [Internet]. 2013 May 16 [cited 2021 Jun 6];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3655441/
- 2. Cox JC, Coulter AR. Adjuvants–a classification and review of their modes of action. Vaccine. 1997 Feb;15(3):248–56.
- 3. Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130433.
- 4. Pati R, Shevtsov M, Sonawane A. Nanoparticle Vaccines Against Infectious Diseases. Front Immunol [Internet]. 2018 [cited 2021 Jun 6];9. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02224/full
- 5. Wang Y, Xia Y. Bottom-Up and Top-Down Approaches to the Synthesis of Monodispersed Spherical Colloids of Low Melting-Point Metals | Nano Letters. Nano Lett. 2004 Sep 2;4(10):2047–50.
- 6. Patra JK, Baek K-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization 7. Techniques. J Nanomater. 2014 Dec 17;2014:e417305.
- 7. Masarudin MJ, Cutts SM, Evison BJ, Phillips DR, Pigram PJ. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol Sci Appl. 2015 Dec 11;8:67–80.
- 8. Abdizadeh H, Vahidshad Y. Influence of Taguchi Selected Parameters on Properties of CuO-ZrO2 Nanoparticles Produced via Sol-gel Method. World Acad Sci Eng Technol Open Sci Index 50 Int J Mater Metall Eng. 2011;5(2):124–32.
- 9. EL-Moslamy SH, Elkady MF, Rezk AH, Abdel-Fattah YR. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci Rep [Internet]. 2017 Mar 28 [cited 2021 Jun 15];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5368611/
- 10. Kim KD, Kim SH, Kim HT. Applying the Taguchi method to the optimization for the synthesis of TiO2 nanoparticles by hydrolysis of TEOT in micelles. Colloids Surf Physicochem Eng Asp. 2005 Mar 10;254(1):99–105.
- 11. Korukonda JR, Korumilli T. Au nanoparticles by instant green chemicals and optimising key synthesis parameters using Taguchi method. Micro Nano Lett. 2019 Sep 25;14(11):1198–203.
- 12. Ibrahim M, Agboola JB, Abdulkareem AS, Adedipe O, Tijani JO. Optimization of green synthesis of silver nanoparticles using Response Surface Method (RSM). IOP Conf Ser Mater Sci Eng. 2020 Jun;805:012022.
- 13. Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci Rep. 2021 Jan 12;11(1):770.
- 14. Othman AM, Elsayed MA, Elshafei AM, Hassan MM. Application of response surface methodology to optimize the extracellular fungal mediated nanosilver green synthesis. J Genet Eng Biotechnol. 2017 Dec 1;15(2):497–504.
- 15. Vakilinezhad MA, Tanha S, Montaseri H, Dinarvand R, Azadi A, Akbari Javar H. Application of Response Surface Method for Preparation, Optimization, and Characterization of Nicotinamide Loaded Solid Lipid Nanoparticles. Adv Pharm Bull. 2018/06/19 ed. 2018 Jun;8(2):245–56.
- 16. Nelson DL, Cox MM. Lehninger principles of biochemistry. 4th ed. W H Freeman & Co; 2004.
- 17. AL-Thabaiti SA, Al-Nowaiser FM, Obaid AY, Al-Youbi AO, Khan Z. Formation and characterization of surfactant stabilized silver nanoparticles: A kinetic study. Colloids Surf B Biointerfaces. 2008 Dec 1;67(2):230–7.
- 18. Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics [Internet]. 2017 Nov 20 [cited 2021 Jun 15];9(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5750659/
- 19. Kvítek L, Panáček A, Soukupová J, Kolář M, Večeřová R, Prucek R, et al. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J Phys Chem C. 2008 Apr 1;112(15):5825–34.
- 20. He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol. 2000 Nov;7(6):899–903.
- 21. He Q, Mitchell A, Morcol T, Bell SJD. Calcium Phosphate Nanoparticles Induce Mucosal Immunity and Protection against Herpes Simplex Virus Type 2. Clin Diagn Lab Immunol. 2002 Sep;9(5):1021–4.
- 22. Joyappa DH, Kumar CA, Banumathi N, Reddy GR, Suryanarayana VVS. Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and guinea pigs against the challenge virus. Vet Microbiol. 2009 Oct 20;139(1–2):58–66.
- 23. Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Controlled Release. 2014 Jan;173:148–57.
- 24. Hong X, Zhong X, Du G, Hou Y, Zhang Y, Zhang Z, et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci Adv. 2020 Jun 1;6(25):eaaz4462.
- 25. Al-Halifa S, Gauthier L, Arpin D, Bourgault S, Archambault D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front Immunol [Internet]. 2019 [cited 2021 Jun 15];10. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00022/full
- 26. Liu X, Qin D, Cui Y, Chen L, Li H, Chen Z, et al. The effect of calcium phosphate nanoparticles on hormone production and apoptosis in human granulosa cells. Reprod Biol Endocrinol. 2010 Apr 2;8(1):32.
- 27. Chen L, Liu J, Zhang Y, Zhang G, Kang Y, Chen A, et al. The toxicity of silica nanoparticles to the immune system. Nanomed. 2018 Aug 1;13(15):1939–62.
- 28. Esmaeili F, Heuking S, Junginger HE, Borchard G. Progress in chitosan-based vaccine delivery systems. J Drug Deliv Sci Technol. 2010 Jan 1;20(1):53–61.
- 29. Margaroni M, Agallou M, Athanasiou E, Kammona O, Kiparissides C, Gaitanaki C, et al. Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFα-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis. Int J Nanomedicine. 2017;12:6169–84.
- 30. Wang Q, Sun X, Huang X, Huang J, Hasan MW, Yan R, et al. Nanoparticles of Chitosan/Poly(D,L-Lactide-Co-Glycolide) Enhanced the Immune Responses of Haemonchus contortus HCA59 Antigen in Model Mice. Int J Nanomedicine. 2021 May 4;16:3125–39.
- 31. Szymańska E, Winnicka K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar Drugs. 2015 Apr 1;13(4):1819–46.
- 32. Garg H, Mehmetoglu-Gurbuz T, Joshi A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci Rep. 2020 Mar 4;10(1):4017.
- 33. Taha MA, Singh SR, Dennis VA. Biodegradable PLGA85/15 nanoparticles as a delivery vehicle for Chlamydia trachomatis recombinant MOMP-187 peptide. Nanotechnology. 2012 Aug 17;23(32):325101.