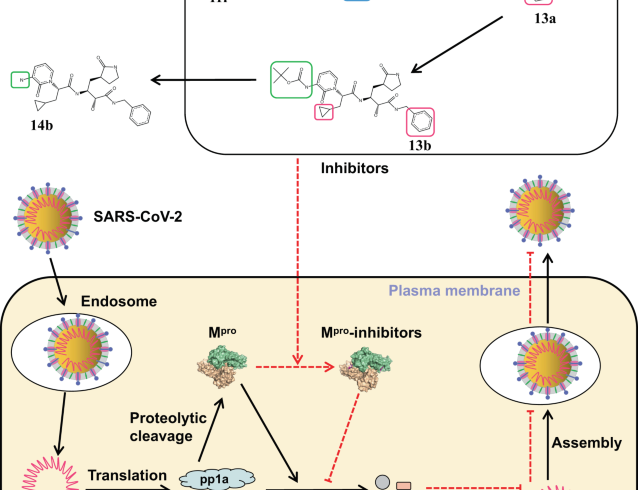
Status of new therapeutics to fight COVID-19
Most of the newly developed COVID-19 vaccines are showing promising results in controlling the pandemic across the globe. However, the hunt for potent and effective therapeutics against COVID-19 is still on because availability of effective therapeutic agent(s) is also a pre-requisite to end the pandemic. In this regard, the world scientific community has identified seven upcoming drugs which are effective against SARS-CoV-2. Brii Biosciences in collaboration with Tsinghua University and Third People’s Hospital of Shenzhen have developed fully human neutralizing monoclonal antibodies, BRII-196 and BRII-198against SARS-CoV-2. Currently, a combination therapy of BRII-196/BRII-198 has entered into its Phase III clinical trial. In another development, a fully human monoclonal antibody, CERC-002 has recently received the Fast Track status from the Food and Drug Administration (FDA). In Phase II trial, it showed promising results against COVID-19 patients having acute respiratory distress syndrome and its efficacy was the highest in COVID-19 patients over 60 years of age having other underlying inflammatory conditions. An engineered anti-human granulocyte-macrophage colony-stimulating factor (GM-CSF) monoclonal antibody viz., Lenzilumab developed by the company Humanigen has been found effective against cytokine storm produced during SARS-CoV-2 infection. It is also currently in its clinical trial. An acellular biologic therapeutic, Zofin has been evaluated in India which showed positive results and 10 patients suffering from moderate to severe COVID-19 were fully recovered. SAB-185, a fully human polyclonal antibody designed by SAb Biotherapeutics showed successful results in early clinical trial and was found to b safe with 25-28 days of half-life. SNG001 (inhaled nebulized interferon-beta-1a) produced by Synairgen cured patients with severe breathlessness problem in its Phase-II clinical trial. Vir Biotechnology and Glaxo SmithKline received EUA from FDA approval for sotrovimab, another monoclonal antibody, to treat COVID-19 patients of 12 years of age or older having mild-to-moderate form of the disease. Besides these, several other therapeutic agents are in different experimental stages or in their early clinical trials.
Source: https://www.genengnews.com/a-lists/seven-up-and-coming-covid-19-drugs/
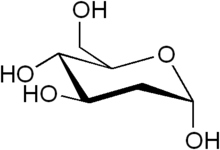
2-DEOXY-D-GLUCOSE (2-DG), A hope in COVID-19 treatment
The Drugs Controller General of India (DCGI) has approved the emergency use of the anti-covid drug 2-DG to treat moderate to severe Covid-19 patients. The drug was developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a laboratory of Defence Research and Development Organisation (DRDO) in collaboration with the pharma company, Dr Reddy’s Laboratories (DRL). The 2-DG is a glucose analogue which accumulates in SARS-CoV-2 infected cells and inhibits the viral synthesis as well as energy production. It accelerates the recovery rate of hospitalized patients and also reduces the supplementary oxygen dependency. The other advantages of this drug are that it can be produced easily in large quantities in powdered form and can be taken orally by just dissolving it in water.
Source: https://www.mpnrc.org/drdo-anti-covid-drug-2dg-medicine-released/

Novel Facemask with SARS-CoV-2 detection technology
Scientists from multi-institute of USA have designed an innovative facemask that can detect SARS-CoV-2 in a patient’s breath. This special facemask is based on wearable freeze-dried cell-free (wFDCF) technology and consists of three unique freeze-dried biological reactions that occur sequentially. The three sequential reactions include breakdown of SARS-CoV-2 membrane and exposure of RNA molecule followed by amplification of spike gene fragment. The third reaction is based on CRISPR-based SHERLOCK technology which reports the detection of the spike gene fragment in patient’s breath via a lateral flow assay strip. This special facemask could detect SARS-CoV-2 within 90 minutes at room temperature and the accuracy of detection was comparable to gold-standard qPCR assay.
Source: 1. Nature Biotechnology (2021). https://doi.org/10.1038/s41587-021-00950-3
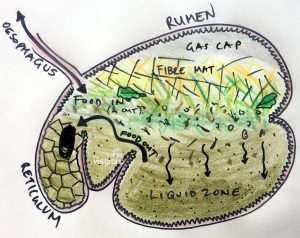
Rumen Microbiome: A Biological Toolbox to degrade Plastic
Plastic is one of the most common and lethal environmental pollutants and it is regarded as one of the biggest threats to nature. Addressing the issue, researchers from Austria have recently conducted a study on rumen content of cattle with respect to plastic hydrolysing properties. From the investigation, it was observed that microbes from cow rumen could digest at least three types of plastics, namely poly(butylene adipate-co-terephthalate) (PBAT), poly(ethylene terephthalate) (PET) and poly(ethylene furanoate) (PEF). Moreover, metagenomics analysis of cow’s rumen revealed that it contains 98% bacteria, 1% eukaryotes, and the other 1% consists of archaea and viral entities.
Source: 1. Frontiers in Bioengineering and Biotechnology (2021). doi.org/10.3389/fbioe.2021.684459
CRISPR gene editing tool has been used to treat internal organs for the first time
An American start-up company, Intellia Therapeutics, recently reported successful treatment of an internal organ for the first time with CRISPR gene editing therapy. The Boston-based start-up company worked in association with the biotechnology company Regeneron to treat a case of thyroxine protein amyloidosis, a devastating disease in which the accumulation of problematic proteins can affect the heart and Nervous system, thereby shortening the life expectancy of a patient.
CRISPR is a system used by bacteria to protect themselves from viruses. Earlier in 2012, Nobel Prize winners Jennifer Doudna, one of the co-founders of the start-up company Intellia Therapeutics along with her French colleague Emmanuelle Charpentier discovered how it can be used as a gene editing tool. Intellia Therapeutics is now working on editing of bone marrow to treat blood-based diseases without transplanting cells. The company is also working with the Bill and Melinda Gates Foundation in an attempt to treat patients with sickle cell anaemia disease in Africa.
Source: 1. Crispr gene editing “revolution” treats internal organs for the first time (quebecnewstribune.com)