Colostral immunity in ruminants
Himasri Das
Ph.D. student (Veterinary Microbiology)
himashridas27@gmail.com
College of Veterinary Science, AAU, Khanapara, Guwahati-781022
The correlation between colostrum and ruminant newborn survival has been deeply characterized. Colostrum contains a complex mixture of proteins that actively participate in the protection of the neonate against various diseases as well as other postpartum environmental challenges. High immunoglobulin concentration in the colostrum ensures passive transfer of antibodies to the newborn, as a result of which the mortality and morbidity are decreased in different neonatal associated diseases such as septicaemia, diarrhoea, respiratory infection, omphalophlebitis etc. The immune system of ruminants becomes completely matured only at 5 to 8 months of age. Although at birth all the essential components of the immune system are present, it takes around two to four weeks to become fully functional. Due to the presence of complexity of the epitheliochorial (cows and water buffaloes) or synepitheliochorial (small ruminants) placentas that separate the maternal and fetal blood, the transmission of immunologic components from the dam to the foetus in utero is prevented. Hence, newborn ruminants are considered to be agammaglobulinaemic (calves) or hypogammaglobulinaemic (lambs and kids) at birth. Therefore, neonatal calves are at high risk of disease immediately after birth. Acquisition of passive immunity through ingestion of colostrum immediately after birth is critical for stimulation of the naive immune system in the ruminant neonates. However, the antibodies are not the sole constituent of colostrum; other maternally derived leucocytes are also available in the colostrum. It contains other substances like antimicrobial and anti-inflammatory agents as well as growth factors that control early gastrointestinal development, cytokines, enzymes and numerous other peptides in higher concentration than in milk. Different milk preparations are reported to be effective in the prevention of several gastro-enteric infections but not in the treatment of an established infection.
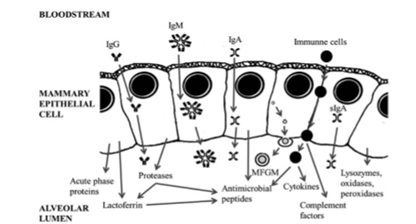
Fig: Schematic representation of the major known proteins involved in the host defence system in colostrum (MFGM: Milk Fat Globule Membrane). [The picture is adapted from Wheeler et al., 2007 and Hernandez et al., 2014].
Migration and trafficking of colostral cells
Upon ingestion of colostrum it is absorbed through the intestinal barrier by the follicle-associated epithelium, i.e. Peyer’s patches of jejunum and ileum within 1.5 to 2 hours and then the colostral cells are trafficked from the neonatal intestine into the circulation which further peaks approximately 24 hours after birth. Both functional as well as phenotypic changes take place in the lymphocytes present in the colostrum such as expression of L- selectin on the surface of the lymphocytes to increase the contact time with blood vessel mucosa hence migration into the blood stream is increased. The absorption of these colostrum components is favoured by low proteolytic activity in the gastrointestinal tract of newborn animals and also by trypsin inhibitors present in colostrum.
The cellular as well as the non-cellular components from the maternal circulation are first transferred into colostrum within the mammary gland through colostrogenesis process. In ruminants, colostrogenesis begins several weeks prior to parturition and concludes near the time of parturition. The immunoglobulins from the dam blood stream first concentrated into the colostrums through the mammary gland epithelium via an active, selective and receptor mediated process. The regulation of this process involves both local and systemic hormonal signalling. The immunoglobulins are first diffused across the endothelium and then attached to the receptors present on the basal membrane of the mammary secretory epithelium. Then they are further taken up by micropinocytotic vesicles that traverse the epithelial cell, and finally secreted into the colostrum. In cattle, IgG subclass, in particular IgG1, is actively present in the colostrum in high concentrations. Therefore, IgG1 is frequently used as marker to see the effectiveness of the passive transfer process. Along with the fat droplets, approximately 106 leukocytes/mL are present in bovine colostrum where macrophages are found to be predominant cell type covering a range of 35 to 79%. In addition to macrophages, milk and colostrum contain lymphocytes and polymorphonuclear and epithelial cells. CD8+ T cells are found to be greater in percentage in the mammary gland of lactating cows whereas CD4+ T cells are predominant in the mammary gland of non-lactating cows. Higher concentration of immunoglobulins has been noted in the colostrum of cows with several lactations.
Immunologic function of colostral protein and cells
A total of three groups of proteins can be found in colostrum. They are located in a complex membrane, called milk fat globule membrane (MFGM). Two of the most important proteins related to immunity are mucin-1 (MUC-1) and xanthine dehydrogenase/oxidase (XDH/XO). Mucin-1 has protective function for the exposed intestinal surfaces both from physical damage and invasive pathogenic microorganisms. In addition, MUC-1 also has the capacity of binding and sequestering pathogenic microorganisms within the gut lumen and participates in the immune reaction in the suckling neonate. XDH provides as a source of H2O2 for lactoperoxidase and hence it acquires antibacterial properties. This protein can act as an immunomodulator that either causes tissue damage or exacerbation of the inflammatory response or induces expression of genes encoding few adhesive proteins, cell receptors, and components of the immune system. There are also some other proteins with important immune functions, particularly –lactalbumin and lactoferrin where lactalbumin acts as an immunomodulator. Lactoferrin functions as an innate immune factor synthesized by the mammary epithelium that provides antimicrobial activity (bactericide and fungicide) both to the mammary gland and the newborn. Four different types of caseins are found in colostrums (αs1, αs2, β and κ) that are responsible for important biological functions such as ion carriers (Calcium, Phosphate, Iron, Zinc, Cupper), bioactive peptide precursors, immunomodulators and also these casein proteolytic fragments have an antimicrobial activity. They stimulate the innate immune system within the mammary gland and prevent udder infections during the dry phase.
The maternal immune cells with already processed antigen present in the colostrum can cross the neonatal intestinal barrier upon ingestion and enter the blood stream. Immune response is triggered against those antigens which might not otherwise be induced due to presence of IgA antibody present in the intestinal mucosa of neonates which capture and eliminate the antigens or it may be due to lack of antigen receptors in the intestinal mucosa. Hence the colostral mononuclear cells modulate the immune responses to those antigens as well as maintain a balance between allergy and immune tolerance. The number of lymphocytes is found to be increased within 6 to 12 hour of ingestion of colostrum and phagocytosis is also found to be efficient in colostrums fed neonates as compared to colostrums deprived neonates. Mode of transfer of passive immunity in the ruminant functions as a possible source of variation in amount of serum immunoglobulins. But the amount of immunoglobulins absorbed at birth can suppress the rate of development of the immune system, in particular for IgG1 production in calves.
In summary, colostrum plays an essential role in several important processes in the newborn, such as PIT (passive immune transfer) and immune system development, decreasing ruminant neonate mortality rates and increasing the economic benefits of farmers. However, colostrum is not only important for its immunoglobulin content but also for the non-immunoglobulin proteins that play a fundamental role in the activation and attraction of immune cells.
References
Besser, T.E. and Gay, C.C. (1994). The importance of colostrum to the health of the neonatal calf. Veterinary Clinics of North America: Food Animal Practice, 10 (1): 107-117.
Bourne, F.J. (1977). The mammary gland and neonatal immunity. Veterinary Science Communications, 1(1): 141-151.
Brandon, M.R.; Watson, D.L. and Lascelles, A.K. (1971). The mechanism of transfer of immunoglobulin into mammary secretion of cows. Australian Journal of Experimental Biology and Medical Science, 49(6): 613-623.
Devery-Pocius, J.E. and Larson, B.L. (1983). Age and previous lactations as factors in the amount of bovine colostral immunoglobulins. Journal of dairy science, 66(2): 221-226.
Hernandez-Castellano, L.E.; Almeida, A.M.; Castro, N. and Arguello, A. (2014). The colostrum proteome, ruminant nutrition and immunity: a review. Current Protein and Peptide Science, 15(1): 64-74.
Gonzalez, D.D. and Dus Santos, M.J. (2017). Bovine colostral cells—the often forgotten component of colostrum. Journal of the American Veterinary Medical Association, 250(9): 998-1005.
LaMotte, G.B. and Eberhart, R.J. (1976). Blood leukocytes, neutrophil phagocytosis, and plasma corticosteroids in colostrum-fed and colostrum-deprived calves. American journal of veterinary research, 37(10): 1189-1193.
Springer, T.A. (1990). Adhesion receptors of the immune system. Nature, 346(6283): 425-434.
Wheeler, T.T.; Hodgkinson, A.J., Prosser, C.G. and Davis, S.R., (2007). Immune components of colostrum and milk – a historical perspective. Journal of mammary gland biology and neoplasia, 12(4): 237-247.
Wilson, R.A.; Zolnai, A.; Rudas, P. and Frenyo, L.V. (1996). T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Veterinary Immunology and Immunopathology, 53(1-2): 49-60.