Lytic bacteriophages induce bacterial cell lysis to release progeny virions from their host cells at the last stage of the lytic cycle. Thus, the bacteriophages have developed several strategies degrading peptidoglycan layers (PGs) which are a major component of bacterial cell walls. For instance, a single lysis gene E of bacteriophage ΦX-174 inhibits murein biosynthesis, and then oligomerizes proteinaceous channels in the cell envelope. Demonstration of the capacity of protein E to effectively inactivate Gram-negative bacteria led to generate genetically inactivated vaccine constructs known as bacterial ghosts (BGs). BGs are empty cell envelopes of Gram-negative bacteria, which have excellent adjuvant and vaccine delivery system properties. BG generated by ΦX174 lysis gene E preserves an intact cell envelope structures containing the potential pathogenic traits of the bacteria, which has the capacity to induce local immunities. Bacterial ghost are non-living gram-negative bacterial cell envelope devoid of cytoplasmic contents, and maintaining their cellular morphology and the surface antigenic structures including their bio-adhesive properties (Won et al., 2017).
Production of Bacterial Ghost
Bacterial ghosts are produced by expression of cloned gene E from bacteriophage PhiX174 resulting in cell lysis in Gram-negative bacteria. Lysis gene E codes for a protein of 91 amino acids and exerts its lytic function in Gram-negative bacteria by the fusion of inner and outer membranes, and transmembrane tunnel formation. Through this tunnel, cytoplasmic content of the bacteria is expelled leaving an empty internal space devoid of the bacterial nucleic acids, ribosomes and other higher or lower lipoproteins, proteins, lipopolysaccharides, DNA and molecular weight constituents, whereas the inner and outer membrane structures are preserved.
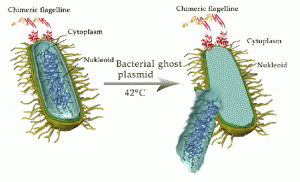
The driving force for the release of cytoplasmic material is the pressure difference between the cytoplasm and the medium created by the opening of the tunnel structure. The current working model of E-mediated lysis divides the process into three phases: Phase 1-: characterized by integration of protein E into the inner membrane, Phase 2-: initiated by a conformational change of protein E transferring its C-terminus across the inner membrane, and Phase 3-: a local fusion of C-terminal domain of protein E towards the surface of the outer membrane of the bacterium (Michael et al., 1996).
Working mechanism
One of the major issues in vaccine designing is finding a safe method to introduce the immunogenic part of the pathogen to the host’s immune system. Besides the safety of the delivery system, it should also be able to present the antigen to the cells of the immune system. Recently, bacterial ghost technology has attracted the attention of vaccine designers, as it has both of these imperative characteristics: safety and efficacy (Jalava et al., 2002).
The key point of using BGs is the especial intact proteins and other pathogen-related compartments on the surface which stimulate immune cells to engulf them immediately when some drugs or a part of another pathogen (e.g. antigen from HIV or influenza virus) are put inside these cell walls or present the desired antigen on the surface of BGs. Immune cells engulf the cell wall which contains some other elements, either inside or at the surface. By this way, direct transfer of target antigen, DNA or drug into the host’s immune cells is possible. Besides the efficient way of delivery, these empty cell walls boost the immune response against the target antigen because of their intrinsic pathogenic characteristics (Langemann et al., 2010).
Several types of vaccines are being researched based on BG technology including vaccines against influenza, HIV, Salmonella and E.coli.
Application in veterinary field
The application of new strategies to develop vaccine is essential in modern veterinary medicine. The bacterial ghost system is a novel vaccine delivery system endowed with adjuvant properties Pigs parenterally immunized with Actinobacillus pleuroneumoniae ghosts were protected against both clinical disease and colonization after challenge. In contrast, parenteral immunization with formalin inactivated bacteria prevented the clinical disease but colonization after challenge was detected. Ghost-vaccinated pigs had a significantly greater antibody titer to a 100-kDa protein than infected convalescent pigs (Huter et al., 2000)
Rabbits and mice were immunized with both Pasteurella multocida and Mannheimia haemolytica ghosts. In rabbit, both specific antibodies to the immunization strain were observed as well as a high degree of cross-reactivity to different serotypes and field isolates of Pasteurella strains were detected (Marchrart et al., 2001).
Bacterial ghosts derived from E. coli O78:K80 strains were prepared and day old chicks were immunized by intramuscular, oral and intranasal routes (Chaudhari et al., 2002). The prospects of Klebsiella pneumonia derived ghosts have been studied and piglets have been immunized with various doses of K. pneumoniae Kpn-3 ghosts with two booster immunizations. The sera from Kpn-3 ghost vaccinated piglets also showed reactivity against human K. pneumoniae strain A565 indicating cross protective immunity with other serotypes (Marchrart et al., 2001).
Salmonella Enteritidis (S. Enteritidis) ghost are promising vaccine candidates because of their immunogenic and enhanced biosafety potential (Kamble et al.,2016). Salmonella typhimurium-based live bacterial vaccine vectors (LBV) exhibited not only the efficient delivery of an adjuvant protein, adhesin and toxin antigens for progressive atrophic rhinitis from an Asd+ plasmid but also showed the significant enhancement of immune response in animal models. The LBVs based on S. Typhimurium can be employed for the delivery of the immunogenic HA1 protein of the influenza virus. The HA1 protein of the influ enza virus is a proteolytically cleaved subunit of the hemagglutinin (HA) integral membrane protein. The HA1 subunit is responsible for the binding of the influenza virus to host cell receptors and is also the principal target for pro tective immune responses in humans and ani mals (Kamble et al., 2017).
Recombinant Bacterial Ghosts
The extended recombinant ghost system is currently evaluated to combining as many as possible candidate vaccines which are stable without the requirement of a cold chain and do not need any adjuvant. For the production of a combination of vaccines against bacterial and viral pathogens or the use of bacterial ghosts as carrier systems for other antigens, a membrane targeting system was developed for the attachment of foreign protein entities to the inner side of the cytoplasmic membrane. By cloning the foreign DNA sequences into the membrane targeting vector, any gene of interest can be expressed as a hybrid protein with N-, C- or N-/C terminal membrane anchors directing and attaching the fusion protein to the envelope complex of the bacteria prior to E-mediated lysis. The membrane-anchored target antigens carried by recombinant ghosts induced humoral as well as a cellular immune responses in animal models. It has been further emphasized that the system of membrane anchoring is not limited by the size of the foreign protein moieties attached to the inside of the inner membrane and that combinations of different antigens to be anchored are possible (Jaleta et al., 2015).
Conclusion
In spite of the exponential rate of discovery of new vaccines resulting from modern molecular biology, the lack of effective delivery technology is a major limiting factor in their application. Bacterial ghosts are very useful non-living carriers as they can carry foreign antigens, nucleic acid and drugs in one or more cellular locations. Their ease of manufacture and the fact that they can be stored and processed without the need for refrigeration and their excellent safety profile-even when administered at high doses-are important considerations for a broad path towards the optimal vaccine development. The identical surface receptors of the bacterial ghost and their living counterparts are being exploited for specific cellular and tissue targeting. Bacterial Ghost is a new technology which has tremendous potential in application as delivery system for vaccines, drugs and nucleic acids, but it needs more work and further investigations before being commercially exploited in a large scale in future.
References:
Chaudhari A., Jawale C. V., Kim S. W. and Lee. J. H. (2012). Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Veterinary Research 43:44. DOI: 10.1186/1297-9716-43-44.
Huter V., Hensel A., Brand E., and Lubitz W. (2000). Improved protection against lung colonization by Actinobacillus pleuroneumoniae ghosts: characterization of a genetically inactivated vaccine, J. Biotechnol. 83: 161–172.
Huter V., Szostak M.P., Gampfer J., Prethaler S., Wanner G. (1999). Bacterial ghosts as drug carrier and targeting vehicles, J. Control. Release 61:51–63.
Jalava K., Hensel A., Szostak M., Resch S. and Lubitz W. (2002). Bacterial ghosts as vaccine candidates for veterinary applications. J Control Release 85:17–25.
Jaleta H., Mamo B. and Disassa H. (2015). Review on Bacterial Ghost and its Application. International Journal of Microbiological Research 6 (3): 200-210.
Kamble N. M., Hyoung K. J. and Lee J. H. (2017). Live-attenuated auxotrophic mutant of Salmonella Typhimurium expressing immunogenic HA1 protein enhances immunity and protective efficacy against H1N1 influenza virus infection. Future Microbiol. 10.2217/fmb-2016-0190.
Kamble N. M., Jawale C. V. and Lee J. H. (2016). Activation of chicken bone marrow-derived dendritic cells induced by a Salmonella Enteritidis ghost vaccine candidate. Poultry Science 10:1–7 http://dx.doi.org/10.3382.
Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. (2010). The bacterial ghost platform system: Production and applications. Bioengineered Bugs. 1(5):326-336. doi:10.4161/bbug.1.5.12540.
Marchrart J. (2001). Pasteurella combination vaccines, Ph.D. Thesis, University of Vienna, Austria.
Michael P., Szostak P., Andreas H., Francis O., Eko F.O., Reinhard K., Tatjana, Horst M., Alexander H., Sebastian B., Gerhard W. and Werner L. (1996). Bacterial ghosts: non-living candidate vaccine. Journal of Biotechnology, 44(1-3): 161-170.
Won G., Hajam I. A. and Lee J.H. (2017). Improved lysis efficiency and immunogenicity of Salmonella ghosts mediated by co-expression of λ phage holin-endolysin and ɸX174 gene E. Scientific Reports doi:10.1038/srep45139.
Rupam Dutta Research Associate, Dept. of Animal Biotechnology, College of Veterinary Science, Khanapara, Guwahati-22.