NEXT GENERATION VACCINE USING NANOTECHNOLOGY
By – Jimon Parashar, Masters in Biotechnology, GITAM University, Visakhapatnam
Vaccines? Gurus of our Immune System
What comes up in our mind when we say the word ‘Vaccine’? Dangerous? Risky? More harm than good? Well, it is inevitable because it is,of course dangerous and risky, and all other words that fill our mind with negativity, and why is this? As learning something new is a kind of violation of our comfort zone and takes time to adapt, our immune system can be imagined similarly. As it feels a little uneasy for us to adapt to changes and the new notion which is put in front of us, it is a case for our immune system as well. However, the conclusion is rather a victory. To understand the victory, let us get to know about the battle and the warriors involved with all the consequences that come along with the fight.
Why Vaccines act as the teachers of our Immune System
Let’s dive into our history when smallpox was more terrifying than a movie today. It is quite disappointing but the origin of smallpox has been lost in the course of history. It is generally believed that it has appeared around 10,000 BC, a time where the first-ever agricultural settlements were observed in Northeast Africa. It was a common knowledge that an individual who survived small box was protected from the infection in future. Mankind was longing for a solution to cure this disease which was referred to as a speckled monster by then. Earlier treatment was of the so-called herbal remedies, cold treatment with special clothes which were implemented to treat or prevent smallpox.
Despite all the efforts, the only successful method before vaccination introduced was inoculation. In simple words, an individual used a lancet (injection) filled with fresh matter of ripe pustule (pimple) from the person who in the past have suffered from smallpox (“Dangerous? Yes it is! But it worked”) and injects (subcutaneous instillation) into a non-immune person.“Well, not everything has only the strengths, isn’t it? What might have been the risk involved for which it leads to hit the brains hard to think of a better way than inoculation? Let’s stop the suspense and move forward…” Risks involved with inoculation ranged from development of disseminated smallpox (“that’s what comes to the mind before injecting the matter from a suffered individual, isn’t it?”) which can spread to others to the transmission of other diseases, e.g. Syphilis through the blood-borne route “And this is dangerous too!”
“So, as you are reading this, I assume, you already know that Edward Jenner discovered vaccination, isn’t it? What if I tell you, it is not the absolute truth? Let’s find out. Let’s go back in the history for a little more and it is very important”. Since smallpox was common, it was relatively known with the word “Variola” which was introduced by Bishop Marius of Avenches. It was derived from the Latin word ‘varius’ which means ‘stained’ or another word ‘varus’ which means ‘marks on the screen’. Inoculation (or variolation) was practiced in Africa, India, and China long before the 18th Century (“Let me remind you, Edward Jenner was from Europe so, let’s dive into Europe”). Variolation came into Europe in about the 18th century which was carried by travelers from Istanbul. The first professionally done variolation was performed in England because of the English aristocrat Lady Mary Wortley Montag. “She is important to remember because she was the only one to introduce variolation in England and well, what made her motivated to perform/initiate the variolation process?”
Because of the loss concerning the family members she faced, she was determined to prevent the ravages of smallpox. She ordered a surgeon to inoculate her 5-year-old son, which was performed in March 1718. “And since everything needed a start, her initiative led to the spread of the practice to several members of the royal family, and slowly the procedure gained its acceptance into the scientific community as well as the society. Now let’s come back to Edward Jenner.”Jenner was born in 1749 and during his school years, he developed a strong interest in science and nature. At the age of 13, he was apprenticed to a country surgeon and apothecary in Sudbury, Bristol. “Now you must be thinking, why these places are important and for some of you who can’t relate, the question strikes why? Because…”It all started in Bristol, where Jenner heard a dairyman say, “I shall never have smallpox for I have cowpox”. It was a common belief back then that, dairymaids were somehow protected from smallpox. For many years, he had been hearing about the tales of dairymaids about how they are somehow protected from smallpox after they had cowpox. Thinking and analyzing from the statements, Jenner concluded that the history of cowpox can protect an individual from smallpox and can also be transmitted between individuals.
“What could be the first-ever case of Edward Jenner for smallpox? Let’s find out…”
May 1796, a time where Edward Jenner found a young dairymaid named Sabrah Nelms and she had fresh cowpox lesions on her hands and arms. He took the materials from her lesions and inoculated into an 8-year-old boy named “James Phipps”. He developed symptoms of cold and fever, and lost appetite but after a few days, he was fine. “Let’s note down the time and month where Jenner inoculated cowpox lesions because…” After 2 months (July 1796), Jenner inoculated the boy again but with fresh smallpox materials. He developed no disease and so Jenner concluded that immunization has been competed.
Brainstorming the word “Vaccination” was done by Edward Jenner
It was the rejection of the paper of the above observations and experiments from the Royal society that made him publish privately a booklet entitled, “An inquiry into the causes and effects of the variolae vaccinae: a disease discovered in some of the western counties of England, particularly Gloucestershire, and known by the name of the cowpox”
Since the Latin word for cow is “Vacca” and cowpox is “Vaccinia”, Edward Jenner decided to call this new procedure “vaccination”. Earlier, it was hard and a struggle for Edward Jenner to find volunteers for vaccination. “Painful rejection, isn’t it? However, he did not give up at all; after all patience is a virtue”. Slowly in London, vaccination became popular through others. Especially through a surgeon named Henry Clive to whom once Jenner had given some inoculants. Finally, Jenner conducted a nationwide survey in search of a proof of resistance to smallpox and the results confirmed his theory. Despite all the struggles, vaccination spread rapidly in England and by the year 1800, it also reached most of the European countries.
So basically, now you know… Jenner’s work was an attempt to the scientific community to control an infectious disease by the use of vaccination. As time passed by, on May 8, 1980, World Health Assembly declared that the world was free from smallpox and recommended all the countries to cease vaccination. Jenner dedicated his life to find the cause of vaccination. He surely gave the “treatment” recognition in the scientific community or it would have been great trouble by now.
The mechanism of Vaccination and its relationship with our Immune system
Let us go to the basics of the Immune system. “So, what does the immune system comprise of? Cells? Molecules? Of course! But how can we remember and recognize them? It surely needs a structure”. To start with, the immune system can be divided into two main categories: The innate immune system and the adaptive immune system. They both interact with each other to provide an effective immune response.“So, the innate immune system is the first defense troops of our army but they are not specific in the field unlike the assassins or sharpshooters with defined targets and missions. They understand about the enemies in the field but not the ones in disguise or the ones who are hiding/not recognizable.” The innate immune system responds to only a specific set of molecular patterns found in all the microorganisms. “Is it why innate immune systems don’t have memory? Particularly yes!” Since immune cells are specific, in other words, they can recognize only conserved molecular patterns of all the microorganisms which means they cannot counter new patterns and create a copy of them, and hence they have no memory.
The innate immune system ranges from anatomic barrier, physiologic barriers, inflammatory response, and pattern recognition receptors to mononuclear phagocytes and granulocytic cells. Innate immune responses can either eradicate the pathogens by themselves or activate adaptive immune responses. “So innate immune system usually rings the headquarters for a backup with special classes of soldiers when things get worse and to deal with specific targets”. Adaptive immune system comprises of B-cells or antibodies and/or T cells. The B-cells invoke humoral/antibody-mediated immunity and T cells are for cell-mediated immunity.
About B-cells in brief
B cells are produced in the bone marrow and then they travel to the lymph nodes. Within the lymph nodes, B cells mature and are exposed to pathogenic agents in a particular lymph node. “We should note that B cells can process antigens in their native form, which means they don’t need another Antigen-presenting cell or T-helper cell” In some cases, activation of B cells with T-helper cells results in the enhanced immune responses and an effective memory, “the goal of immunization”. The B cell receptor has Fab-region where the antigen binds and the secondary signaling from the cytokines released from the T-helper cells hyper-mutates the Fab region for adjusting and tightening the antigen-Fab region leading to the maturation to plasma cells and begins to produce a particular antibody which is a correct and relevant fit to the antigens.“Now those specified B cells will be cloned and some of them will be assigned two types of duties; One of the duties is to actively produce plasma cells for rapid antibody production and another to become memory cells while the cycle can continue again upon next interaction”.
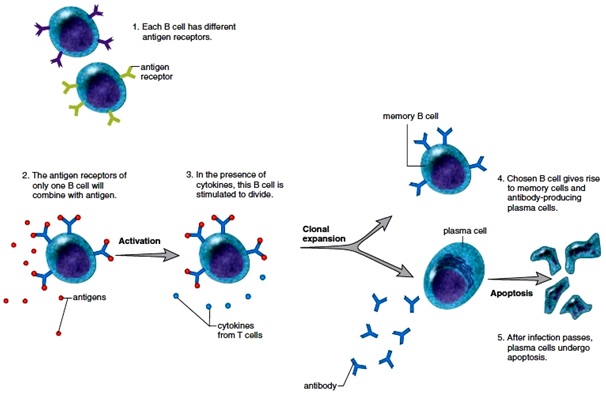
Figure : The clonal selection of B cells. Source:www.encyclopedia.lubopitko–bg.com
IgM is the first antibody produced during clonal selection and as it progresses, the activated plasma cell will produce IgG which is a larger antibody. An antigen binds more efficiently to IgG and it aids in opsonization. Other types of antibodies include IgD, IgA, and IgE but the main important antibody is IgG.
What about the Cell-mediated Immunity?
Cell-mediated immunity functions primarily against intracellular pathogens. The T-cells mature in the thymus and are then released into the stream. There are two types of T-cells CD4, “also known as T helper cells” and CD8, “also known as cytotoxic T cells”
CD4 cells interact with MHC II molecule (Major histocompatibility II) and CD8 cells interacts with MHC I molecules which is present in all the nucleated bodies. The T cells need the antigens to be processed and presented by Antigen Presenting Cells. There are two types of T cell responses, intracellular and extracellular. The intracellular antigens bind with MHC I molecules which are in the case of viral and tumor cells, whereas extracellular antigens bind with MHC II which is in the case of bacteria and parasitic agents. When T cells are activated, depending on CD4 or CD8, it goes clonal expansion and produces effector and memory T cells.
A little into the immunity by a vaccine
In brief, the body must recognize and identify if an object is a pathogenic agent or a part of immunization, which is initially done by the innate immune system and in some cases, the B cells. The immune system recognizes the epitopes on the antigens. “Epitopes – the marker regions on the antigens which can elicit recognition”. Different components of the innate immune system will respond to this challenge and will be engulfed by the antigen-presenting cells (APCs) like the macrophages or monocytes. APCs will then process the antigens from the pathogenic agent and will insert them to the surface of the APC with MHC protein tagged with it. If it is a viral antigen, it will be bound to MHC I protein and presented by APC to CD8 cells (Cell-mediated immunity), whereas if it is a bacterial antigen, it will bind to MHC II and APC to CCD4 cells for humoral immunity.
The types of Vaccines available today
- Live Attenuated Vaccine: It contains a weakened pathogenic agent and is produced in the laboratory. Since it is a weakened version of the original pathogen, it produces long-term immunity. The main disadvantage is that it has the possibility of reversion to the original virulent form of the pathogen, which is a very dangerous consequence. Examples of the diseases against which live attenuated vaccines are used include Measles, mumps, and chickenpox.
- Inactived Vaccine: A process where the pathogenic agent is killed or inactivated by chemicals, heat, or radiation treatment, which results in vaccines being stable but it produces a weaker immune response and so, additional booster shots are required.
- Subunit Vaccine: It includes only the epitopes or antigenic determinants of the pathogen that most readily stimulate the immune system. Since epitopes are specific, it becomes hard to determine the antigen to be included in the vaccines. Examples include the Hepatitis B vaccine.
- Conjugate Vaccine: A procedure where antigens or toxoids from microbes are linked to polysaccharides from the outer coating of the microbe to stimulate immunity. Examples include vaccines for Haemophilia influenza type B.
- Toxoid Vaccine: It is produced by inactivating bacterial toxins with formalin. It stimulates immune response against bacterial toxins. Examples include Diphtheria, Tetanus toxoid.
- f. Naked DNA/RNA Vaccine: It uses DNA/RNA specific for microbial antigens to stimulate immunity. This will be injected into the body cells and they would start producing the antigens to stimulate immunity.
- Recombinant vector vaccine: Uses either an attenuated virus or microbe to introduce microbial DNA into the body cells.
Modern vaccines include mRNA, DNA, recombinant protein vaccines, etc. “But how nanotechnology is related to vaccines? Yes, there is an answer to this but let’s go through nanotechnology fast”
What nanotechnology is and why?
It is the engineered device at the micro-molecular level in the range of a nanometre. “One nm is one millionth of a meter… Pretty small, isn’t it? Well, you can imagine DNA strand being
2 nm wide”. Why things are more interesting in the nanoscale? Guess what! The answer is in the size and its surface area. Lower the size higher is its surface area, which results in greater bioavailability and also the action of a particular substance will be higher because more surface area is exposed. It is very interesting that the nanomaterial is ultimately very small and can easily enter the cell which is why it led to the development of many biological applications especially in the field of drug discovery, drug delivery, and gene/protein delivery. Drug consumption and the side effects can be lowered by targeting the active agent in the desired location.“So, how does nanotechnology help? Guess right! It particularly helps in the route of administration!”
Route of administration – A way of nanotechnology into vaccines
- Oral Route: The development of polymer-based nanoparticles is useful in the delivery of oral DNA vaccines (Bhasar and Anjii, 2007). The main drawback is the dilution during the passage of entering the gastrointestinal tract and hence a higher concentration is required to work.
- Nasal Route: Makidon and his team in 2008 showed nanoemulsion with hepatitis B antigens which have been reported to be a safe and effective hepatitis B vaccine. (Interesting, but how the emulsion is made?) Surprisingly, it is made up of soybean oil (the ones which are common at times), alcohol, water, and detergents where they are simply emulsified into droplets of 40 nm. Furthermore, conventional vaccines need 3 shots however the nanoformulation needs only 2 shots. It is also reported to be safe and effective with lesser side effects because of lower doses and it cannot be used like alum (Alum is an adjuvant that is at risk of causing irritation and discomfort). Intranasal delivery has two challenges – the first is that repeated dispensing of a very small quantity of formulated vaccines to all the areas of nasal mucosa while limiting the deposition of particles in the lungs (Sharma et al. 2009). Flumist vaccine for influenza is an example that is commercialized. As stated, the main problem is the delivery since the free antigens are readily cleared from the nasal cavity.
- Intradermal route: Since it is challenging to deliver drugs and genes through the skin because of its tough layers into the underlying epidermal cells which are immunologically sensitive. The drug is emulsified with an adjuvant and then it forms a depot to allow slow and controlled release of the drug over a longer duration.
There are three types of adjuvants available: particulate ones (The ones with oil emulsions), non-particulate ones (Such as saponins and combined combinations). The role of adjuvants is to slowly release the antigens to the relevant antigen-presenting cells or directly activating the cells of the immune system.
Sinyakov and his group in 2006 used biodegradable magnetic nanoparticles (mnP ~ 100-150 nm) and mono-dispersed polyacrolein nanoparticles (pdNP ~200 nm) and inserted the submicron polymeric particles as an adjuvant which were covalently bound to BSA. Compared to conventional adjuvants, the BSA-paNP was superior and it was comparable to a conventional adjuvant – BSA Alum.“This tells us that the use of nanotechnology to evoke an immune response is much safer, faster and effective. It can act as an adjuvant and in a controlled way. The prospects of nano-vaccine are high but it needs more research to engineer. Moreover, it is being investigated with great interest by the scientists today.”
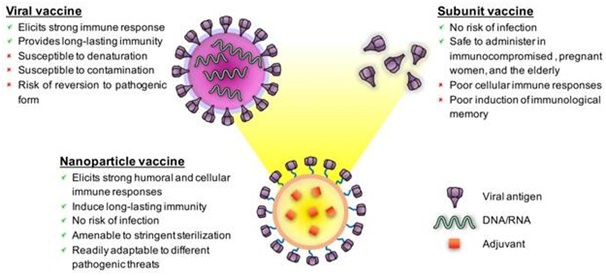
(Figure: Schematics of a viral vaccine, a subunit immunization, and a representative nanoparticle vaccine featuring the qualities and deficiencies of each platform. Nanoparticle vaccines can be designed to impersonate viruses concerning morphology, antigen display, and adjuvanticity. Source: Chattopadhyay et al., 2017)
“Interesting enough? Let us see how nanotechnology contributes to the recent pandemic of the year 2020”.
Nanotechnology and it’s breakthrough in the development of COVID19 vaccine
Commonly, the utilization of nanoparticles in immunization could satisfy three unique purposes:
- Stability of antigens and protecting them against the premature degradation from the proteolytic enzyme.
- Enhancement in immunogenicity because it can act as an adjuvant which is an immune-stimulant.
- Targeted delivery of the antigens for uptake and processing ability of antigen-presenting cells.
Nonetheless, of late motivated by the possibility of virus-like nanoparticles, researchers equipped self-assembling protein nanoparticles (1c-SApNPs) with SARS CoV2 protein spikes as stated by statnano.com. This framework can incite the immune system to quickly produce antibodies to kill (or deactivate) the coronavirus eventually offering a beneficial assurance against the real SARS-CoV-2 infection.
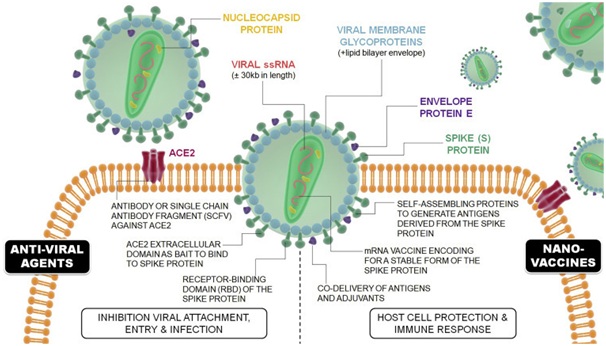
(Therapeutic Approaches by which nanotechnology can contribute against COVID-19. Nanovaccines, associated with host cell protection and immune and immune response, and against anti-viral agents, associated with hindering viral attachment, cellular entry, and infection. Image source: Talebian and Conde, 2020)
Usage of mRNA vaccines is for real. But why?
Since the enormous production of viral proteins related to these antibodies carries with it a few difficulties including the requirement for a manufacturing process that is adaptable and financially savvy. To address these inadequacies, new vaccines based on messenger RNA have been proposed. These immunizations give a synthetic mRNA of the virus for which the host can utilize it to produce antigenic viral proteins itself.
Moderna, a clinical-stage biotechnology organization spearheading mRNA therapeutics is as of now testing an mRNA-based vaccine candidate (mRNA-1273) against COVID-19. mRNA 1273 encodes a stabilized form of the spike protein and is embedded in lipid nanoparticles (in statnano.com). Because of the instability of the mRNA molecule, lipid nanoparticle is used to encapsulate the mRNA vaccine. Moreover, on the improvement of nanovaccines, Novavax, a late-stage biotechnology organization creating cutting edge new generation vaccines for serious infections declared that it has build up a coronavirus vaccine candidate, NVX-CoV2373, a stable and profoundly immunogenic prefusion protein made inserted on Matrix-M adjuvant to upgrade immune reactions and invigorate significant degrees of neutralizing antibodies.
These nanovaccines are models concerning how nanotechnology can additionally improve the therapeutic impact of COVID-19 vaccines.
Biomimetic nanovaccines, another technique in the field of nanotechnology and vaccines:
Despite the conventional vaccines as discussed earlier, Biomimetic nanoparticles have novel antigenic attributes and immune-stimulatory properties and have been utilized to plan more formulation of a successful vaccine. This methodology straightforwardly utilizes the standards of a common plan to produce multifunctional and multi-antigen nanosystems that may assume significant usage soon. Biomimetic nanoparticle vaccines typically comprise three components: viral antigen molecules, adjuvants, and a protein platform. The viral antigens actuate the adaptive immune system, and the adjuvants improve the innate immune response.
Current biomimetic nanoparticles accessible for vaccine development include virosomes, VLPs, self-collecting protein nanoparticles, and engineered nanoparticles, with sizes going from 10 to 200 nm. VLPs (Virus-like particles) are the most often utilized biomimetic nanoparticles in vaccine development. VLPs contain both antigens and adjuvants, and prime immune cells to prompt immune reactions after inoculation.
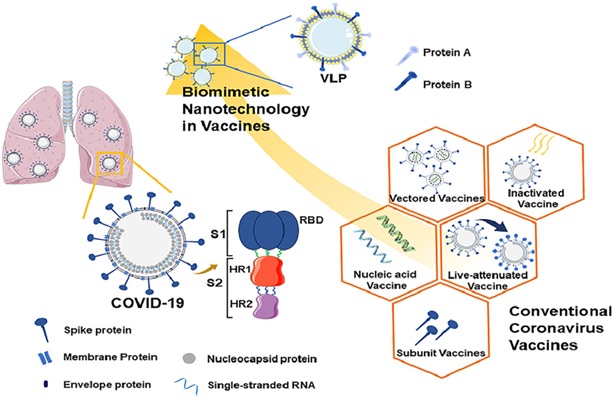
(Figure: Covid vaccine advancement. Coronavirus is encoded by single-stranded RNA and comprises spike proteins, nucleocapsid proteins, envelope proteins, and membrane proteins. Among them, spike proteins are made out of S1 and S2 subunits, and the receptor-binding area is situated on the S1 protein.The image shows Covid vaccine improvement from regular antibody to biomimetic nanotechnology antibody. Source: Huang et al., 2020).
At present, there are several vaccines available for COVID-19, but for development of a vaccine with increased efficacy, there is a wide scope of exploring the emerging field of science and innovation like nanotechnology, which may help in prevention, diagnosis and treatment of COVID-19 in the near future.
References:
- Clem, A.S. (2011). Fundamentals of vaccine immunology. Glob. Infect. Dis. 3(1):73-8. doi: 10.4103/0974-777X.77299. PMID: 21572612; PMCID: PMC3068582.
- Nandedkar, T.D. (2009). Nanovaccines: recent developments in vaccination. Biosci. 34(6):995-1003. doi: 10.1007/s12038-009-0114-3. PMID: 20093753.
- Iwasaki, A and Omer, S.B. (2020). Why and How Vaccines Work. Cell. 183(2):290-295. doi: 10.1016/j.cell.2020.09.040. PMID: 33064982; PMCID: PMC7560117.
- Talebian, S. and Conde, J. (2020). Why Go NANO on COVID-19 Pandemic? Matter. 3(3):598-601. doi: 10.1016/j.matt.2020.08.005. PMID: 32905308; PMCID: PMC7466941.
- Chattopadhyay, S.; Chen, J.Y.; Chen, H.W. and Hu, C.M.J. (2017). Nanoparticle Vaccines Adopting Virus-like Features for Enhanced Immune Potentiation. Nanotheranostics, 1(3):244-260. doi:10.7150/ntno.19796.
- Huang, L.; Yuan, R.; Qin, P.; Kezhen, Y.; Xuan, T.; Qian, Z.; Wei, W.; Jianyuan, W. and Fubing, W. (2020). SARS-CoV-2 vaccine research and development: Conventional vaccines and biomimetic nanotechnology strategies. Asian Journal of Pharmaceutical Sciences. doi: 10.1016/j.ajps.2020.08.001