Odonata richness in RAMSAR site, Deepor Beel, Assam, India
Ankita Dey¹* and Debojit Dey²
- Department of Microbiology, Assam University, Silchar – 788011 Assam, India
- Department of Health & Family Welfare, National Centre for Vector Borne Disease Control,
Karimganj -788710 Assam India
* Corresponding Author
Email: dey_ankita@outlook.com
Abstract:
The study was conducted in the Deepor beel wetland for 18 months from January 2015 to mid june 2016. The Deepor beel is a RAMSAR site located (longitude 910 38 35 E and latitude 260 07 30 N) in the Kamrup district of Assam and is owned by the State Fisheries Department. Odonates are boon for the environment. They act as a biological indicator of a healthy aquatic ecosystem and also act as a biocontrol agent, as adult odonates prey on flies and mosquitoes which are parasites and pests of men and animals. During the present study, eight Anisopteran (Family: Libellulidae) and three Zygopteran (Family: Coenagrionidae ) species could be found in and around the Deepor beel wetland site. Shurb land was found to be the most favourable habitat for the odonates. The present findings showed that the study area is biologically active to contain divergent species of odonate fauna. However, a detailed environmental study is necessary so that the habitat of the odonates could be protected and conserved in situ against the increasing level of pollution, as less number of divergent species were recorded in the present study compared to previous surveys.
Keywords: Ramsar, Deepor beel, Odonates, Anisopteran, Zygopteran, Assam
Introduction
The order Odonata comprises of dragonflies and damselflies. The Order constitutes a small but a well known group of insects, which are widely distributed over the world (Tillyard, 1917). During the post-monsoon months, the dragonflies and the damselflies are found in abundance in and around the vicinity of natural ponds, lakes and other standing as well as flowing water bodies in India. The adults when in rest or in flight display their beauty and elegance, their splendor of colors and their countless numbers. The odonates can be well-differentiated from occasionally confused specimen of ant lions and mayflies. Dragonflies have almost minute and invisible antenna, which are very much conspicuous in ant lions and mayflies.
A popular myth surrounds the dragonflies as being an insect which stings. However, in reality the odonates are perfectly harmless organisms. In fact, the distal end of the abdomen contains accessory sexual appendages, which apparently look like sting apparatus similar to honeybee’s sting. It is interesting to note that the odonates have great flying power and do possess no defense organs. Odonates constitute a part of aquatic ecosystems. Their distribution ranges from temporary to permanent water bodies (Corbet, 1999).
Adult odonates prey on flies and mosquitoes as well as smaller moths which are considered to be parasites and pests of men and animals. Hence, they are also considered as Biological pest control agents. Fraser (1933) commented that without a vast number of dragonflies and damselflies found in the Indian subcontinent, which act as scavengers, the life in the subcontinent would have been unbearable.
Odonates are predatory insects and are active during the day time (Moore, 1997). Some species do also fly after dark or at dusk which live entirely on mosquitoes (Fraser, 1933). There are fossil records of odonates of more than 300 million years old, implying that the dragonflies lived as long as dinosaurs. Therefore, it was concluded that they must have appeared during the Carboniferous era (Subramanian, 2005).
Odonates are very sensitive to environmental conditions for which these groups of insects make an excellent biological indicator of environmental condition (Brown, 1991). Odonates have few enemies such as birds, lizards, fishes, frogs and spiders. Moreover, sundews (Drosera peltata) have also been incriminated to catch dragonflies. There are some dragonflies (Onthetrum sabina) which exclusively feed upon their own kind, i.e. they are cannibals.
Fraser (1933) described that dragonflies often attacked by some species of small Hymenopterans (Tetragrammidae and Myramaridae) deposit their eggs on those of dragonflies. Symbiotic relationship between dragonflies and small red mites (a hydrachnid) has also been observed. The red mites often cling to wings, legs and thorax of certain species of dragonflies, e.g. Ceriagrion coromandelianum.
Dragonflies also have migratory habits like butterflies, but this habit is confined to a few species of dragonflies, e.g. Pseudagrion decarum, P. microcephalum and Acagrion occidentale. For migration, larger species depend on their own flight power. However, a smaller and weaker species depend entirely on upper air currents. Migration usually takes place in an east to west direction.
Dragonflies generally remain within the boundary of their watery birth places. After emergence, though they wander for distances, yet they return to their former homes for the purpose of breeding. Generally males do not depart from such places and wait for the return of the females. Some odonates use to make isolated colonies. They are found inhabiting in the same localities over many decades. When the breeding areas like tanks, lakes and marshes dry up during drought, the endemic species also perish therewith. However, as old colonies are dried up, entirely new group of species replace the habitat in tandem. The availability of dragonflies has a direct ratio to the rainfall or water supply to any particular breeding site. As far as the Indian subcontinent is concerned, the Western Ghats, the southern slopes of Himalayas, the alluvial flood plain of the river Brahmaputra and Barak and also the moisture and wet tracks of the nearby Myanmar and Sri Lanka are by far the richest in odonata biodiversity. In addition, some of the drier zones of the Indian subcontinent also have their distinctive odonate fauna. Though dragonflies have great flying powers, yet they are mostly locals. Certain species are confined to very short tracts of their breeding place.
Dragonflies are very seasonal insects. Fraser (1933) indicated that the best months for collection of dragonflies are May, June and September to the first half of November. This implies that the first two months preceded the south-west monsoon and later two months follow the south – east monsoon. Commonly the species are found in the air. The richest odonates fauna is found at an altitude zone of 2000-3500 ft above sea level.
During the breeding season, the adult males establish territory along the wetland. The sexually mature and receptive female visits territories along with male. The breeding habitat includes both flowing and stagnant water bodies; they have specific habitat requirements. After matting, the female lays eggs in water. The egg hatches and larvae emerge in water. In the life history of odonates, there are three stages- the egg, the larvae (nymphal stage) and imago. They have no pupal or resting stage, but the change from larval to imaginal life is direct and there occurs a change in the structure at metamorphosis. The aquatic life is longer and the aerial life is shorter. The aquatic life of larvae varies greatly in length. The smaller species spend about three months in aquatic environment (Lestes). However, the larger species take from 1 to 3 years to reach maturity. Both larvae and adult are predacious and feed on their own kind, often tadpoles, small fishes and larvae of many flies. Like dragonflies, the damselflies are also predacious, hemi-metabolous and amphibiotic insect. Nearly all dragonflies possess what are known as recognition males, i.e. a pale colored spot on the terminal segments of the abdomen, which is apparently used as a guide for the opposite sex.
Damselflies have narrow rectangular head and their eyes are separated. Both the hind and forewings of damselfly are similar in shape, size and venation. Wings are narrow toward the base leading to the formation of stalk in certain families. When they sit, the wings are held together over the slender abdomen or only separated slightly. Like dragonflies, damselflies are also important as a bio-control agent being predacious in food habit.
The Indian odonates have largely derived from Malaysian and to a less extent from Palearctic zone (Fraser, 1933). The Western Ghats of India has rich biodiversity fauna of odonates. Odonates found in the east and north eastern region of India, e.g. the states of Assam, West Bengal, Arunachal Pradesh and Bihar have pronounced Malaysian affinities. Scientific literature on the species biodiversity, habitat preference, abundance etc. of the odonates in Assam and the North East as well is very much limited barring a few works (Nair, 2011; Borah et al., 2012 ; Papari et al., 2012; Barua and Saikia, 2015 ; Das et al., 2015; Kalita and Roy, 2015).
The most comprehensive study on Indian dragonflies during the colonial period was done by Fraser (1933), which described the species found in the British India including Ceylon (now Sri Lanka) and Burma (now Myanmar). His description also included species prevalent in Assam.
Baruah and Saikia (2015) studied the abundance and diversity of odonate species in different habitats of Barpeta district of Assam. They could record a total of 45 species of odonata which included 29 species under 3 families of Anisoptera and 16 species different families of Zygoptera from four different habitats in Barpeta district during the year 2012 and 2013. A total 38 species were found near ponds and rivers, 39 species were recorded near hillls and 41 species were recorded from open tracks. The most abundant Anisopteran species were Diplacodes trivalis in ponds, Rhythemis variegata variegata from hills and rivers and Pantala flavescens from open tracts. Of the Zygoptera species, the most abundant species was Ceriagrion coromandelianum in all the habitats under study.
The diversity, distribution and abundance of damselflies (Zygoptera) in beels of Barpeta district Assam was studied by Kumar et al. (2015). The study was carried out for a calendar year from June 2013 to May 2014. A total 26 species of damselflies belonging to 3 families of 11 genera were identified. Of 26 species, 88.46% belonged to the family Coenogrionidae followed by Platyenemididae with less species diversity of the family Calopterygidae.
Kalita and Roy (2015) made a study on the diversity and habitat preference of odonates in Deepor beel Bird Sanctuary located in Kamrup district of Assam. A total 39 species belonging to five families and 22 genera were recorded. The species Ceriagrion tibiae and Agrinemis kalinga were recorded for the first time from Assam. The Family Libellulidae (Sub-order Anisoptera) was the most dominant family under the Sub-order Anisoptera. The species Rhyothemis variegata was the most abundant species under the Sub-order Zygoptera.
From the foregoing information, it is apparent that odonates have immense importance in ecology. They also act as biological indicator of a healthy aquatic ecosystem. As there is not much information available on the odonate fauna in the Deepor beel, an important wetland ecosystem, the present study was undertaken to explore the species available in and around this particular wetland to add scientific information over the existing one.
Materials and Methods:
Study area:
Description of the study area
The study was conducted in the Deepor beel wetland for 18 months from January 2015 to mid june 2016. The Deepor beel wetland is located (longitude 910 38 35 E and latitude 260 07 30 N) in the Kamrup district of Assam owned by State Fisheries Department (Fig.1 & Fig.2). It’s a fresh water lake (Fig.3) on the southern bank of river Bramhaputra, which covers an area of about 900 hectares (full water spread area) otherwise its area coverage is 589 hectare. The beel is situated at an altitude of 53msl. Geographicaly, it’s a freshwater swamp, where annual rainfall is more than 2000 millimeter and temperature varies from 7 to 37 degree centigrade. The study site is a Wildlife Sanctuary established in January 1989. It is also a RAMSAR site (designated in November, 2002). The main source of water in this lake is from rainfall and runoff water from the Bashistha and Kalamoni rivers. The water from this swamp drains in to the river Brahmaputra located about 5 kilometers away through a small rivulet known as Khanajan. The southern side of the lake is continuous with the Rani Reserve Forest. About half of the beel dries out during the winter and the exposed shores are converted into paddy fields (Fig.2).
Biodiversity of the study area
A large variety of aquatic macrophytes of tropical wetland is found in abundance in the study site along with its adjoining areas. The dominant aquatic macrophytes are Azolla piñnata (xaru puni), Nymphaea rubra (bhet), Ottelia alismoides, Eleocharis plantaginea (pani singora), Pistia stratiotes, Hydrilla verticellata (Hydrilla), Potamogeton crispus (bon), Ipomoea reptans (kolmou), Sagittaria sagittifolia, Nymphaea albea (bhet), Vallisneria spiralis, Euryale ferox (giant water lily) and Eichhornia crassipes (pani meteka). Among these macrophytes, E. crassipesis is the dominant species found in this wetland. The diversity and concentration of indigenous freshwater fish species in this wetland is very high (around 50 species under 19 families). It harbors many species of migratory birds during the winter season as well as there are many resident birds (Fig.4) too. About 150 species of birds have been recorded so far in and around this Sanctuary including nine threatened species. This wetland is used for fishery (Fig.4), domestic water supply, food and fodder supply, wildlife study (Fig.4), transport and recreation (Fig.4). Presently heavy siltation, pollution (Fig.2), poaching, and unregulated fishing (Fig.4) and encroachment have been reported in this wetland. Moreover, the wetland area is fragmented surrounded by water logged area in a 5 km buffer zone.
Method of study
The study was conducted once in a week in a line transect of 50 m length on the shore of the wetland. In situ observation was done on the adult specimen of both the Anisopteran and Zygopteran specimens which perched on the stems or leaves of aquatic macrophytes or on the open land. Photographs were taken and specimens were collected using a fly catching net. The collected specimens were subjected to taxonomic identification using the standard key of Fraser (1933). Digital photographs were taken and compared with similar materials published in peer-reviewed journal and interpreted.
Taxonomy
There are approximately 6000 species with 600 genera, 8 super families, 29 families, and 58 sub-families of dragonflies described from all over the world (Silsby, 2011). India contains 470 species under 139 genera and 19 families of odonates. The Order Odonata can be classified into two Sub-Orders i.e. Zygoptera, which contains the damselflies and the Anisoptera the true dragonflies (Fraser, 1933, 1936). Since 18th century, the odonate species are being studied in India. During the pre-independence period, various scientists, for example, Selys – Longchamps, Laidlaw and Fraser have contributed significantly on the odonate species in India (Subramanian, 2009). In Zygoptera, eyes are well separated, fore and hind wings are approximately same in shape and breadth. In Anisoptera, the eyes are usually confluent across the middle line or at the most very slightly separated. Fore and hind wings are of variable in shape and the hind pair of wings is usually considerably broader at the base than the forewings.
Identification criteria
The morphology of eggs, larvae (nymph) and the adult are used for identification of these species. The adults can be identified comparatively easily than the nymphal stages. For identification of the adults, the entire body is divided into head, thorax and abdomen.
Head
The head contains two large compound eyes which are either fused broadly across the middle line (Anisopteta). In addition, there are three tiny ocelli situated between and in the front of the Anisoptera species; whereas, they are found in groups in small eminences known as the vesicles. They have short inconspicuous filliform antennas, which are composed of 4-7 segments. The mouth parts are biting types, mandibles with strong incisor and molar teeth, maxilla with simple unjointed palp and robust is toothed internal lobe. Labium is short and very broad made up of a median and two lateral lobes.
Thorax
The thorax is composed of a small pro-thorax which forms a neck and a larger mass formed from the fused meso and meta thorax also known as synthorax. By means of the pro-thorax, the insect is able to move its head freely in different direction. The pro-thorax consists of an anterior lobe, collar like and narrow, a middle lobe forming the greater part of the structure and a posterior lobe which is important for the purpose of classification. The shape of this lobe is different in all the genera and also in the species. This lobe is often armed with hooks or spines on its hinder border in the females and more rarely in the males also. The synthorax is very oblique so that the anterior surface comes to be the dorsum, carrying back the wings and thrusting the leg forwards beneath the head.
Leg
They have three pairs of legs which are not used for walking but are formed for clinging and catching the prey. To catch the prey, the legs are bunched together forming an open basket. The interstices of legs are closed in by long spines situated on the femur rebus. The structure on tibia, femur and coxa are used for taxonomic identification.
Wings
The wings are in pair; in zygoptera, the wings are held vertically over thorax and abdomen, while in anisoptera they are open widely and horizontally. The membrane is hyaline in nature or partly opaque. It may be coloured or uncoloured and may contain hair or scales but has numerous fine spines on the under surface of the supporting ribs or nerves of the wings. The wing venation, for example, the old Elysian System, is more a nomenclature than annotation. The other system is Comstock-Needham which is better than the earlier one. There is another notation called Tillyard’s notation, which is generally used for classification. The classification of dragonflies is largely dependent on variation of structure if wings and its venation.
Pterostigma is a thickened chitinized variable shaped cell situated on the coastal margin near the apices of the wing. It may be absent in one or other wing or in one of the sexes or it may differ in shape in fore- and hind- wings in male, and lastly may be braced or not at its proximal end. Other parts of the wings are coastal (C), nodal base (N), space, nerves, discoidal cell (DC), sub node (SN), anti nodal nerves (AN), median space (NS), sub-median or cubital space (CS), anal bridge(AB) and anal crossing (AC).
Abdomen
In Zygoptera, the abdomen is elongated and cylindrical. In Anisopteta, the abdomen is broad, flattened, constricted or fusiform. The abdomen consists of 10 segments. The anal appendages are attached to the last segment.
Anal appendages:
In Zygoptera, the male contains both paired superior and inferior appendages. In Anisoptera, there is a pair of single inferior appendages.
Genitalia:
The male genitalia is situated on the ventral surface of second segment of the thorax.
Results and Discussion
Suborder: Anisoptera (Dragonflies)
In the present study, eitght different Anisopteran species could be recorded in the Deepor beel wetland, which belonged to the family Libellulidae. The species and their habitat patterns have been presented in Table 1. The species identified were Trithermis pallidinervis Kirby (1889), Orthetrum sabina Drury (1770), Diplacodes trivialis Rambur (1842), Brachydiplax chalybea Brauer (1868), Brachythermis contaminata Fabricius (1793), Rhodothemis rufa Ris. (1909), Neurothermis fulvia Drury (1773) and Brachydiplax sobrina Rambur (1842).
Table 1. Species of Anisopterans under the family Libellulidae at Deepor beel wetland along with habitat preferences
| Family | Zoological name | Habitat* |
|
Libellulidae |
Trithermis pallidinervis Kirby (1889) Fig.5 | SB;TG;EV |
| Orthetrum sabina Drury (1770) Fig.6 | SB;SG;TG;OA;EV;FFV | |
| Diplacodes trivialis Rambur (1842) Fig.7 | SB;SG;OA | |
| Brachydiplax chalybea Brauer (1868) Fig.8 | SB;EV;FFV | |
| Brachythermis contaminata Fabricius (1793) Fig.9 | SB;SG | |
| Rhodothemis rufa Ris. (1909) Fig.10 | SB | |
| Neurothermis fulvia Drury (1773) Fig.11 | SB;SG;TG;OA;EV;FFV | |
| Brachydiplax sobrina Rambur (1842) Fig.12 | EV ; FFV |
* SB: Shrub land; SG: Short grass land; TG: Tall grass land; OA: Open area; EV: Emergent vegetation; FFV: Free floating emergent vegetation.
Damselflies (Suborder: Zygoptera)
In the present study, three different species of damselflies could be recorded in the Deepor beel wetland which belonged to the Family Coenagrionidae. The list of species along with the habitat pattern of the species is presented in Table 2. The species identified were Agriocnemis lacteola Selys. (1877), Ceriagrion cerinorubellum Brauer (1865) and Onychargia atrocyana Selys (1865).
Table 2. Species of Zygopterans under the family Coenagrionidae at Deepor beel wetland along with habitat preferences
| Family | Zoological name | Habitat* |
|
Coenagrionidae |
Agriocnemis lacteola Selys. (1877) Fig.13 | SG; EV |
| Ceriagrion cerinorubellum Brauer (1865) Fig.14 | SB, OA, SG, EV, FFV | |
| Onychargia atrocyana Selys (1865) Fig.15 | SB,OA,SG, EV,FFV |
* SB: Shrub land; SG: Short grass land; OA: Open area; EV: Emergent vegetation; FFV: Free floating emergent vegetation.
During the present study, eight Anisopteran (Family: Libellulidae) and three Zygopteran (Family : Coenagrionidae ) species could be found in and around Deepor beel wetland site. Previously, Kalita and Ray (2015) could record 39 species which belonging to five families and 22 genera of dragonflies. All the species that were recorded in the present study were also recorded by Kalita and Ray (2015). However, the less number of divergent species were recorded in the present study. The present findings showed that the study area is biologically active to contain divergent species of odonate fauna; however, it is under stress causing a gradual decline in the species diversity. A detailed environmental study is necessary so that the habitat of odonates could be protected and conserved in situ against the increasing level of pollution.
Acknowledgement:
Authors express special thanks to Professor Dr. Saidul Islam, Department of Parasitology, College of Veterinary, Assam Agricultural University- 785013 for providing laboratory facilities, identification of species, taking photographs, conceptualization, writing, editing and supervision for successful completion of the present research work.
Bibliography:
Baruah, C. and Saikia, P.K. (2015). Abundance and diversity of Odonates in different habitats of Barpeta district, Assam, India. Int. Res. J. Biol. Sci., 4(9): 17-27.
Borah, P., Acharjee, B.K., Das, M., and Saikia, P.K. (2012). Diversity and distribution of damselflies in Gauhati University campus, Assam, India, NeBIO, 3(2): 33-36.
Brown, K. S. J. (1991). Conservation of Neo Tropical Environment; insect as indicator, In; Collins N.M . and J.A Thomas (eds). Conservation of Insects and Their Habitats, Academic Press. New York.
Corbet, T. S.(1999). Dragonfly: Behaviour and Ecology of Odonata. Cornell University Press, New York.
Das, B.K., Sarma, U. and Chetia, P. (2015). Diversity, distribution and abundance of damselflies (Zygoptera) of Kapla beel, Wetland of Barpeta district: Assam, India. Int. Res. J.Biol. Sci., 4(4):69-76.
Fraser, F.C. (1933). The Fauna of British India including Ceylon and Burma. Odonata Vol. I., Today and Tomorrow’s Printers and Publishers, New Delhi.
Fraser, F.C. (1936). The Fauna of British India including Ceylon and Burma. Odonata Vol. III., Today and Tomorrow’s Printers and Publishers, New Delhi.
Kalita, G.J. and Ray S.D. (2015). Studies on the diversity and habitat preference of odonates in Deepor Beel bird Sanctuary, Kamrup , Assam. J. Ento. Zool. Studies, 3(2): 278-285.
Nair, M.V. (2011). Dragonflies and Damselflies of Orissa and Eastern India. Wildlife organization, Forest and Environment Department, Govt. of Orissa.
Papari et al.(2012). Diversity and distribution of damselflies in Gauhati
University campus, Assam, India. NeBIO, 3(2):32-36.
Silsby, J . (2011). Dragonfly of the World. Natural History Museum in Association with CSIRO Publishing, UK.
Subramanian, K. A (2009). A Checklist of Odonata (insect) of India. Zoological Survey of India.
Subramanian, K. A.(2005). Dragonfly and Damselfly of Peninsular India – A field Guide. Project Life Scape. Indian Academy of Sciences, Bangalore, India.
Tillyard, R. J. (1917). The Biology of Dragonfly. Cambridge University Press.
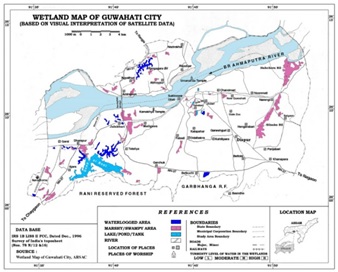
Fig.1. Wetland map of greater Guwahati showing location of Deepor beel (Analogue map by courtesy of Assam Remote Sensing Application Centre©,Guwahati, Assam)
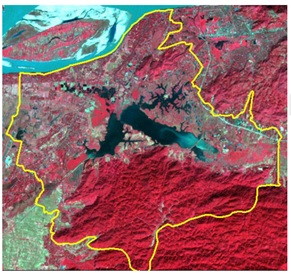
Fig.2. Satellite remote sensing imagery showing Deepor beel wetland area during 2002. Deep reddish colour towards south shows forested areas. Light reddish colour around the beel shows open vegetation (cultivation) and macrophytes. Light bluish colored area on the south-east side of the beel shows siltation. (Satellite Imagery by courtesy of Assam Remote Sensing Application Centre©, ISRO, Guwahati)

Fig.3. Bird’s eye view of apart of the Deepor beel wetland.

Fig.4. Representative fauna and flora with different human activities in the Deepor beel wetland

Fig.5. Trithermis pallidinervis Kirby (1889)

Fig.10. Rhodothemis rufa Ris. (1909)

Fig.11. Neurothermis fulvia Drury (1773)

Fig.6. Orthetrum sabina Drury (1770)

Fig.7. Diplacodes trivialis Rambur (1842)

Fig.12. Brachydiplax sobrina Rambur (1842)

Fig. 13. A male Agriocnemis lacteola Selys. (1877)

Fig.8. Brachydiplax chalybea Brauer (1868) (Female)

Fig.9. Brachythermis contaminata Fabricius (1793)

Fig.14. A male Ceriagrion cerinorubellum Brauer (1865)

Fig.15. A Male Onychargia atrocyana Selys (1865)