Shyamalima Saikia, Minakshi Puzari and Pankaj Chetia*
Molecular Plant Taxonomy and Bioinformatics Research Laboratory
Department of Life Sciences, Dibrugarh University, Dibrugarh-786004, Assam
*Corresponding author email: chetiapankaj@dibru.ac.in
Abstract
Antimicrobial resistance (AMR) is a major global public health concern. AMR adversely affects clinical and therapeutic outcomes, resulting in treatment failures, elevated morbidity and mortality rates, longer hospital stays, and increased healthcare expenses.Antibiotic resistance in bacteria occurs via diverse resistance mechanisms and primarily stems from the overuse and misuse of antibiotics in healthcare and agriculture. The emergence of resistance to all available antibiotics, particularly among critical bacterial pathogens, underscores the urgent need for novel antimicrobial discovery efforts. Understanding the molecular mechanisms of resistance is crucial for devising strategies to mitigate its emergence and dissemination, as well as for developing innovative therapeutic approaches against multidrug-resistant (MDR) species. A multifaceted approach is neededto prevent the spread of AMR, and optimizing antibiotic use is the need of the hour. This article highlights the current scenario of antibiotic resistance and summarizes issues faced in combating resistance in bacteria.
Keywords:Antibiotics; Antibiotic resistance; Antibiotic stewardship; β-lactamases; Enterobacteriaceae
Introduction
The discovery of antibioticsstands as one of the most significant breakthroughs of the twentieth century (Hutchings et al., 2019; Cook and Wright, 2022). Among antimicrobials, antibiotics are considered super drugs, with their revolutionary impact beginning with the discovery of penicillin in 1928(Gould, 2016). These life-saving therapeutic agents have revolutionized modern medicine by curing many infectious diseases and facilitated various medical interventions such as surgeries, organ transplantation, and the treatment of cancer and autoimmune diseases (Fair and Tor, 2014; Cook and Wright, 2022). Unfortunately, easy access, low cost, and minimal adverse effects of antibiotics have contributed to the emergence of bacterial resistance, which occurs through various mechanisms (Davies and Davies, 2010). Consequently, infections caused by some common bacterial pathogens have become untreatable, even under antibiotic pressure(Cook and Wright, 2022).
Antimicrobial resistance is one of the most pressing public health emergencies jeopardizing the effective treatment of numerous infections caused by various pathogens, including bacteria, viruses, and fungi (Darby et al., 2023). AMR occurs when bacteria acquire the capability to resist the lethal effects of antibiotics, rendering infections they cause more challenging to treat (Blair et al., 2015). Such resistance not only amplifies the risk of severe illness and death but also exacerbates the spread of disease, which can impact individuals of any age at any stage of life (Fair and Tor, 2014). As per reports, AMR caused 1.27 million deaths worldwide in 2019 alone, and it is estimated that it will claim the lives of more than 10 million people by 2050 if it remains unsolved (Uddin et al., 2021). In the United States (US), over 2.8 million cases of AMR infections are reported annually. Low- and middle-income countries (LMICs) are more vulnerable to AMR and face a higher risk of its effects (Uddin et al., 2021). India is among the worst hit countries, witnessing a rise in resistance to nearly all last-resort antibiotics. This poses a growing threat with a high burden of infectious diseases and massive antibiotic consumption (Kaur et al., 2024).The World Health Organization (WHO) declared AMR as one ofthe top ten public health emergencies(World Health Organization, 2017). In 2001, the WHO introduced the Global Strategy for Containment of Antimicrobial Resistance, providing a framework for interventions to slow the emergence and mitigate the spread of AMR. The challenges posed by AMR, particularly in bacteria, underscore the critical need for developing control strategies and new antibiotics that can effectively combat resistance in them (Kaur et al., 2024). This article describes the global incidence and the burden of bacterial infections, as well as challenges to combat antibiotic resistance in bacteria.
Arrival of the superbugs: the global scenario
Bacteria that have developed resistance to antibiotics are commonly referred to as superbugs (Khan and Khan, 2016). Among these, the bacteria resistant to antibiotics of at least three different classes are considered multidrug-resistant (MDR), while those non-susceptible to all antibiotics except two or fewer classes are termed extensively drug-resistant (XDR) (Magiorakos et al., 2012). Consequently, bacterial isolatesresistant to antibioticsof all classes are known as pan-drug-resistant (PDR). According to the Centre for Disease Control and Prevention (CDC), the ESKAPE pathogens- Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. need special attention as theyare responsible for most hospital and community-acquired infections (Venkateswaran et al., 2023). Additionally, these pathogens are more prone to drug resistance due to their genetic plasticity and ability toacquire multiple resistance determinants,conferring resistance to nearly all antibiotics and leaving limited options for treatment (Mulani et al., 2019). The issue of increasing antimicrobial resistance is further exacerbated by the limited number of novel antimicrobial agents currently in development (Cook and Wright, 2022).
The rising antibiotic resistance has numerous consequencessignificantly impacting economy, society, food safety, and public health. Currently, an average of 700,000 people worldwide die annually from drug-resistant infections (Miller and Arias, 2024). This number is projected to increase to 4.73 million in Asia, 4.15 million in Africa, 0.392 million in Europe, and 0.317 million in North America annually by 2050, resulting in a global economic loss of $100 trillion (Murray et al., 2022; Kaur et al., 2024). India ranks among the top countries with a significant burden of infectious diseases, reporting 65,561 cases of drug resistance out of 100,000 samples collected in 2019 (ICMR, 2021). According to the annual report 2021 by the Indian Council of Medical Research (ICMR), the susceptibility of K. pneumoniae to imipenem declined from 65% in 2016 to 43% in 2021(Kaur et al., 2024). Similarly, the susceptibility of Escherichia coli to imipenem decreased from 86% in 2016 to 64% in 2021. The ICMR report also highlighted that E. coli and K. pneumoniae isolates resistant to carbapenems also exhibited resistance to other antimicrobials, posing challenges in the treatment of carbapenem-resistant infections (ICMR, 2021). Moreover, it was observed that 87.5% of the studied A. baumannii isolates exhibited resistance to carbapenems, thereby limiting the available treatment options. Among the Gram-positive bacteria, Staphylococcus spp. is responsible for 65.5% of infections, followed by Enterococcus spp. (17.5%) and Streptococcus spp. (7.1%) (ICMR, 2021). These Gram-positive pathogens are highly resistant to vancomycin, penicillin, erythromycin, ampicillin, and ciprofloxacin; however,they remain sensitive to gentamicin and tetracycline, except for methicillin resistant Staphylococcus aureus (Murray et al., 2022). In 2022, among the 107,053 culture-positive cases of drug-resistant pathogens, the highest number of infections were recorded for E. coli, followed by K. pneumoniae, P. aeruginosa, A. baumannii, and S. aureus (ICMR, 2022). Among the tested E. coli isolates, 39% were producers of CTX-M-15 β-lactamase, whereas SHV was identified as the most common β-lactamase in K. pneumoniae, occurring in 55% of the isolates (ICMR, 2022). Resistance to carbapenems in A. baumannii was found to be 87.8%, with the prevalence of blaOXA-23-like carbapenemase detected in 76% of the isolates. Among the tested isolates causing bloodstream infections in intensive care units, 88% of A. baumannii and 75% of K. pneumoniae isolates were imipenem-resistant (ICMR, 2022).
In 2017, the WHO published its first bacterial priority list by mapping the global burden of drug-resistant infections (WHO, 2017). However, the threat AMR poses has intensified over the past seven years. Recently, WHO released an updated priority list 2024, identifying 15 families of drug-resistant bacteria, categorized into critical, high, and medium priority for developing new therapeutic agents to combat AMR (WHO, 2024). The critical priority pathogens include carbapenem-resistant A. baumannii and Enterobacteriaceae, third-generation cephalosporin-resistant Enterobacteriaceae (including K. pneumoniae), and rifampicin-resistant Mycobacterium tuberculosis. Carbapenem-resistant P. aeruginosa, S. aureus, fluoroquinolone-resistant Salmonella, and Shigella are categorized as high-priority pathogens because of their high burden in LMICs (WHO, 2024).
Mechanisms of antibiotic resistance
Antibiotics can be categorized into several classes based on their targets on the bacterial cell. The primary drug targets include components involved in cell wall synthesis, nucleic acid synthesis and function, protein synthesis, metabolic activity, and other related factors (Uddin et al., 2021). The bacterial cell wall is the primary attractive target for the majority of antibiotics, such as β-lactams, including penicillins, cephalosporins, monobactams, and carbapenems (Hutchings et al., 2019; Darby et al., 2023). All β-lactam antibiotics and their derivatives inhibitpeptidoglycan biosynthesis by targeting the penicillin-binding proteins (PBPs), making the bacterial cell vulnerable to osmotic pressure and autolysis(Breilh et al., 2013).
Bacteria develop resistance to antibiotics through various mechanisms, which can be either intrinsic or acquired. Intrinsic resistance involves changes in the bacterial genome due to repeated exposure to antibiotics, while acquired resistance arises through gene mutation and acquisition of resistance genes via horizontal gene transfer (HGT) between similar or different bacterial species (Uddin et al., 2021; Darby et al., 2023). The primary mechanisms by which bacteria develop resistance include the production of hydrolytic enzymes, overexpression of efflux pumps, porin down regulation, target alteration, and utilization of alternate metabolic pathways (Blair et al., 2015). Bacteria produce various hydrolytic enzymes, such as β-lactamases, which inactivate the β-lactam antibiotics by hydrolyzing them. Certain antibiotic modifying enzymes, such as transferases, transfer acyl, phosphate, nucleotidyl, ribityl, glycosyl and thiol groups to antibiotics, thereby preventing the antibiotic binding to their respective targets (Uddin et al., 2021; Darby et al., 2023). Examples of such enzymes include aminoglycoside-modifying enzymes, which confer high levels of resistance to aminoglycoside antibiotics (such as gentamicin, amikacin, tobramycin)through group modification (Blair et al., 2015; Darby et al., 2023).
Among the hydrolytic enzymes, β-lactamases are the most common cause of resistance, which can be divided into four main classes: class A, class B, class C, and class D β-lactamases. Class A β-lactamases, also known as Extended Spectrum β-lactamases (ESBLs), confer resistance to all penicillins, cephalosporins, and monobactams (Ambler, 1980; Brown and Amyes, 2006; Rezaee et al., 2013). Metallo-β-lactamases (MBLs) are zinc-dependent class B β-lactamases associated with high levels of carbapenem resistance in bacteria. The expression of these resistance genes encoding different β-lactamases can be significantly enhanced when bacteria are repeatedly exposed to antibiotic pressure, resulting in extreme resistance to the antibiotic (Uddin et al., 2021). Besides enzyme-mediated antibiotic resistance, efflux pumps play a crucial role in conferring resistance in bacteria by expelling antibiotics out of the bacterial cell, thereby reducing the intercellular concentration of the antibiotic to sub-lethal levels (Darby et al., 2023).
Drivers of antibiotic resistance and challenges
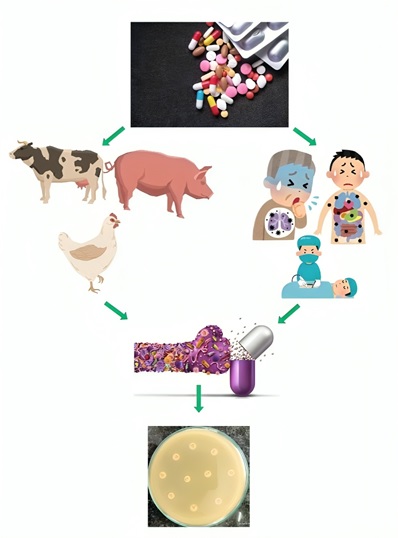
AMR poses a significant concern, and the incidence rate is gradually mounting. The emergence of antibiotic resistance in bacteria primarily stems from the irrational use and overuse of antibiotics (Vikesland et al., 2019). Notably, while antibiotics are employed to control human diseases, they are frequently used as growth promoters in livestock farming, poultry, and aquaculture (Holmes et al., 2016).India stands as a major producer and consumer of antibiotics. Antibiotics are easily accessible in India and LMICs, often without the need for a prescription (Laxminarayan and Chaudhury, 2016). This effortless availability results in extensive and often unnecessary usage, even when not medically required.Several studies have highlighted that the substantial antibiotic consumption in India is a major driver of AMR (Fazaludeen Koya et al., 2022; Skender et al., 2024). Reports indicate that antimicrobial consumption in India surged by 12.0% from 2011 to 2016, with the defined daily dose rising from 4749 to 5358 million and decreasing to 5071 million in 2019 (Fazaludeen Koya et al., 2022). In 2017, WHO introduced the Access, Watch, and Reserve (AWaRe) classification to promote the judicious use of antibiotics. Despite this initiative, India experienced a 24.7% increase in antibiotic consumption from 2011 to 2019, indicating rising levels of AMR (Skender et al., 2024).
Furthermore, the absence of regulations concerning antibiotic use in animal husbandry has contributed to the emergence of more resistant bacteria (Laxminarayan and Chaudhury, 2016). Numerous studies have identified MDR and XDR Enterobacteriaceae, including E. coli, K. pneumoniae, and Citrobacter spp., in chicken meat samples (Amer et al., 2013; İnat et al., 2023). Additionally, a high prevalence of antibiotic-resistant pathogenic bacteria in milk has been reported from various parts of the world (Tóth et al., 2020; Hassani et al., 2022). Moreover, there needs to be more public awareness and understanding regarding the appropriate use of antibiotics in India. Many individuals do not complete the full antibiotic course prescribed, potentially leading to the development of resistance to these medications (Laxminarayan and Chaudhury, 2016). Poor infection control measures in healthcare settings are another contributing factor, resulting in elevated rates of hospital-acquired infections. The practice of prescribing broad-spectrum antibiotics instead of narrow-spectrum ones is another reason for exacerbating the burden of AMR (Holmes et al., 2016).
The environment plays a crucial role in the transmission of AMR. A significant portion of antibiotics remains incompletely degraded, leading to the presence of antibiotic residues in soil and wastewater (Larsson and Flach, 2022). This, in turn, facilitates the dissemination of antibiotic-resistant bacteria, antibiotic-resistant genes, and antibiotic residues, turning the environment into a significant reservoir of AMR, further contributing to the spread of more resistant pathogens. This linkage underscores the urgency to address the environmental dimensions of the escalating AMR crisis (Larsson and Flach, 2022).
Although AMR remains a significant concern in India, comprehensive surveillance reports covering all regions of the country are still lacking, which complicates efforts to understand the extent of the issue and design appropriate interventions. Although the Indian Council of Medical Research (ICMR) has issued guidelines for hospital infection control and the rational use of antibiotics, the widespread practice of selling antibiotics persists in the country. While reliable data on the incidence of MDR bacterial infections are lacking, thousands of new cases are diagnosed each year (Murray et al., 2022). Bacterial infections caused by MDR M. tuberculosis, Helicobacter pylori, P. aeruginosa, A. baumannii, Enterobacteriaceae members, and Neisseria gonorrhoeae are becoming progressively challenging to treat as bacteria have developed or acquired resistance to last-resort antibiotics. As a consequence, patients experience prolonged hospital stays, necessitating the use of costlier antibiotics and facing elevated rates of morbidity and mortality from the infecting bacteria (Fazaludeen Koya et al., 2022; Skender et al., 2024).
Conclusion
Addressing the spread of AMR is an urgent global concern. To combat this issue, a multifaceted approach is needed to prevent the spread of antibiotic-resistant bacteria, including information on the current burden of bacterial AMR worldwide, prevalent pathogens, effective drug combinations, infection control measures, vaccination, and surveillance. Education of healthcare providers and the public on appropriate antibiotic use is crucial, along with implementing antibiotic stewardship programs in healthcare settings to reduce antibiotic overuse.Additionally, the development of new antibiotics capable of counteracting resistance mechanisms and rapid diagnostic tests arealso important to guide proper antibiotic prescribing. Addressing this growing threat requires global collaboration and the adoption of the “One Health” approach, which involves multiple disciplines working together to promote optimal health for people, animals, and the environment while preventing the spread of AMR.
References:
Ambler, R.P. (1980). The structure of β-lactamases. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, Vol. 289 No 1036, pp.321-331.
Amer, M.M., Dahshan, A.H.M., S. Hassan, H. and A. Mohamed, A. (2013). Studies on the Prevalence of Enterobacteriaceae in Chickens and Chicken eggs, Journal of Veterinary Medical Research, Vol. 22 No. 1, pp. 136–144, doi: 10.21608/jvmr.2013.77696.
Blair, J.M.A., Webber, M.A., Baylay, A.J., Ogbolu, D.O. and Piddock, L.J.V. (2015). Molecular mechanisms of antibiotic resistance, Nature Reviews Microbiology, Vol. 13 No. 1, pp. 42–51, doi: 10.1038/nrmicro3380.
Breilh, D., Texier-Maugein, J., Allaouchiche, B., Saux, M.-C. and Boselli, E. (2013). Carbapenems, Journal of Chemotherapy, Vol. 25 No. 1, pp. 1–17, doi: 10.1179/1973947812Y.0000000032.
Brown, S. and Amyes, S. (2006). OXA β-lactamases in Acinetobacter: the story so far, Journal of Antimicrobial Chemotherapy, Vol. 57 No. 1, pp. 1–3, doi: 10.1093/jac/dki425.
Cook, M.A. and Wright, G.D. (2022). The past, present, and future of antibiotics,Science Translational Medicine, Vol. 14 No. 657, p. eabo7793, doi: 10.1126/scitranslmed.abo7793.
Darby, E.M., Trampari, E., Siasat, P., Gaya, M.S., Alav, I., Webber, M.A. and Blair, J.M.A. (2023). Molecular mechanisms of antibiotic resistance revisited, Nature Reviews Microbiology, Vol. 21 No. 5, pp. 280–295, doi: 10.1038/s41579-022-00820-y.
Davies, J. and Davies, D. (2010). Origins and Evolution of Antibiotic Resistance, Microbiology and Molecular Biology Reviews, Vol. 74 No. 3, pp. 417–433, doi: 10.1128/MMBR.00016-10.
Fair, R.J. and Tor, Y. (2014). Antibiotics and Bacterial Resistance in the 21st Century, Perspectives in Medicinal Chemistry, Vol. 6, p. PMC.S14459, doi: 10.4137/PMC.S14459.
Fazaludeen Koya, S., Ganesh, S., Selvaraj, S., Wirtz, V.J., Galea, S. and Rockers, P.C. (2022). Antibiotic consumption in India: geographical variations and temporal changes between 2011 and 2019,JAC-Antimicrobial Resistance, Vol. 4 No. 5, p. dlac112, doi: 10.1093/jacamr/dlac112.
Gould, K. (2016). Antibiotics: from prehistory to the present day, Journal of Antimicrobial Chemotherapy, Vol. 71 No. 3, pp. 572–575, doi: 10.1093/jac/dkv484.
Hassani, S., Moosavy, M.-H., Gharajalar, S.N., Khatibi, S.A., Hajibemani, A. and Barabadi, Z. (2022). High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk, Scientific Reports, Nature Publishing Group, Vol. 12 No. 1, p. 3878, doi: 10.1038/s41598-022-07845-6.
Holmes, A.H., Moore, L.S.P., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Guerin, P.J., et al. (2016). Understanding the mechanisms and drivers of antimicrobial resistance, The Lancet, Elsevier, Vol. 387 No. 10014, pp. 176–187, doi: 10.1016/S0140-6736(15)00473-0.
Hutchings, M.I., Truman, A.W. and Wilkinson, B. (2019). Antibiotics: past, present and future, Current Opinion in Microbiology, Vol. 51, pp. 72–80, doi: 10.1016/j.mib.2019.10.008.
Indian Council of Medical Research (2021). Annual Report: Antimicrobial Resistance Research and Surveillance Network, January 2021-Dec 2021, Indian Council of Medical Research.
Indian Council of Medical Research(2022). Annual Report: Antimicrobial Resistance Research and Surveillance Network, January 2022-Dec 2022, Indian Council of Medical Research.
İnat, G., Sırıken, B., Çiftci, A., Erol, İ., Başkan, C. and Yıldırım, T. (2023). Molecular characterization of extended-spectrum β-lactamases-producing Enterobacteriaceae species in ground beef and chicken meat, International Journal of Food Microbiology, Vol. 398, p. 110228, doi: 10.1016/j.ijfoodmicro.2023.110228.
Kaur, J., Singh, H. and Sethi, T. (2024). Emerging trends in antimicrobial resistance in bloodstream infections: multicentric longitudinal study in India (2017–2022), The Lancet Regional Health – Southeast Asia, Elsevier, Vol. 26, doi: 10.1016/j.lansea.2024.100412.
Khan, S.N. and Khan, A.U. (2016). Breaking the Spell: Combating Multidrug Resistant ‘Superbugs, Frontiers in Microbiology, Vol. 7, p. 174, doi: 10.3389/fmicb.2016.00174.
Larsson, D.G.J. and Flach, C.-F. (2022). Antibiotic resistance in the environment, Nature Reviews Microbiology, Nature Publishing Group, Vol. 20 No. 5, pp. 257–269, doi: 10.1038/s41579-021-00649-x.
Laxminarayan, R. and Chaudhury, R.R. (2016). Antibiotic Resistance in India: Drivers and Opportunities for Action, PLOS Medicine, Vol. 13 No. 3, p. e1001974, doi: 10.1371/journal.pmed.1001974.
Magiorakos, A.-P., Srinivasan, A., Carey, R.B., Carmeli, Y., Falagas, M.E., Giske, C.G., Harbarth, S., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance.Clinical Microbiology and Infection, Elsevier, Vol. 18 No. 3, pp. 268–281, doi: 10.1111/j.1469-0691.2011.03570.x.
Miller, W.R. and Arias, C.A. (2024). ESKAPE pathogens: antimicrobial resistance, epidemiology, clinical impact and therapeutics, Nature Reviews Microbiology, doi: 10.1038/s41579-024-01054-w.
Mulani, M.S., Kamble, E.E., Kumkar, S.N., Tawre, M.S. and Pardesi, K.R. (2019). Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review.Frontiers in Microbiology, Vol. 10, p. 539, doi: 10.3389/fmicb.2019.00539.
Murray, C.J., Ikuta, K.S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A., Han, C., et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis, The Lancet, p. S0140673621027240, doi: 10.1016/S0140-6736(21)02724-0.
Rezaee, M.A., Pajand, O., Nahaei, M.R., Mahdian, R., Aghazadeh, M., Ghojazadeh, M. and Hojabri, Z. (2013). Prevalence of Ambler class A β-lactamases and ampC expression in cephalosporin-resistant isolates of Acinetobacter baumannii, Diagnostic Microbiology and Infectious Disease, Vol. 76 No. 3, pp. 330–334, doi: 10.1016/j.diagmicrobio.2013.04.003.
Skender, K., Machowska, A., Dhakaita, S.K., Lundborg, C.S. and Sharma, M. (2024). Ten-year trends of antibiotic prescribing in surgery departments of two private sector hospitals in Central India: a prospective observational study, BMC Public Health, Vol. 24 No. 1, p. 310, doi: 10.1186/s12889-024-17817-2.
Tóth, A.G., Csabai, I., Krikó, E., Tőzsér, D., Maróti, G., Patai, Á.V., Makrai, L., et al. (2020). Antimicrobial resistance genes in raw milk for human consumption, Scientific Reports, Nature Publishing Group, Vol. 10 No. 1, p. 7464, doi: 10.1038/s41598-020-63675-4.
Uddin, T.M., Chakraborty, A.J., Khusro, A., Zidan, B.R.M., Mitra, S., Emran, T.B., Dhama, K., et al. (2021). Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects, Journal of Infection and Public Health, Vol. 14 No. 12, pp. 1750–1766, doi: 10.1016/j.jiph.2021.10.020.
Venkateswaran, P., Vasudevan, S., David, H., Shaktivel, A., Shanmugam, K., Neelakantan, P. and Solomon, A.P. (2023). Revisiting ESKAPE Pathogens: virulence, resistance, and combating strategies focusing on quorum sensing, Frontiers in Cellular and Infection Microbiology, Vol. 13, p. 1159798, doi: 10.3389/fcimb.2023.1159798.
Vikesland, P., Garner, E., Gupta, S., Kang, S., Maile-Moskowitz, A. and Zhu, N. (2019). Differential Drivers of Antimicrobial Resistance across the World, Accounts of Chemical Research, American Chemical Society, Vol. 52 No. 4, pp. 916–924, doi: 10.1021/acs.accounts.8b00643.
World Health Organization. (2017). Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities, World Health Organization, Geneva.
World Health Organization. (2024). WHO Bacterial Priority Pathogen List, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance, World Health Organization, Geneva.